Clinical, cellular, and molecular aspects in the pathophysiology of rosacea
- PMID: 22076321
- PMCID: PMC3704130
- DOI: 10.1038/jidsymp.2011.7
Clinical, cellular, and molecular aspects in the pathophysiology of rosacea
Abstract
Rosacea is a chronic inflammatory skin disease of unknown etiology. Although described centuries ago, the pathophysiology of this disease is still poorly understood. Epidemiological studies indicate a genetic component, but a rosacea gene has not been identified yet. Four subtypes and several variants of rosacea have been described. It is still unclear whether these subtypes represent a "developmental march" of different stages or are merely part of a syndrome that develops independently but overlaps clinically. Clinical and histopathological characteristics of rosacea make it a fascinating "human disease model" for learning about the connection between the cutaneous vascular, nervous, and immune systems. Innate immune mechanisms and dysregulation of the neurovascular system are involved in rosacea initiation and perpetuation, although the complex network of primary induction and secondary reaction of neuroimmune communication is still unclear. Later, rosacea may result in fibrotic facial changes, suggesting a strong connection between chronic inflammatory processes and skin fibrosis development. This review highlights recent molecular (gene array) and cellular findings and aims to integrate the different body defense mechanisms into a modern concept of rosacea pathophysiology.
Conflict of interest statement
The authors state no conflict of interest.
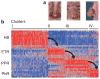
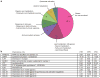
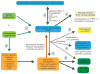
Figures



References
-
- Abram K, Silm H, Maaroos HI, et al. Risk factors associated with rosacea. J Eur Acad Dermatol Venereol. 2010a;24:565–71. - PubMed
-
- Abram K, Silm H, Oona M. Prevalence of rosacea in an Estonian working population using a standard classification. Acta Derm Venereol. 2010b;90:269–73. - PubMed
-
- Akamatsu H, Oguchi M, Nishijima S, et al. The inhibition of free radical generation by human neutrophils through the synergistic effects of metronidazole with palmitoleic acid: a possible mechanism of action of metronidazole in rosacea and acne. Arch Dermatol Res. 1990;282:449–54. - PubMed
-
- Aloi F, Tomasini C, Soro E, et al. The clinicopathologic spectrum of rhinophyma. J Am Acad Dermatol. 2000;42:468–72. - PubMed
-
- Aroni K, Tsagroni E, Kavantzas N, et al. A study of the pathogenesis of rosacea: how angiogenesis and mast cells may participate in a complex multifactorial process. Arch Dermatol Res. 2008;300:125–31. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

