Magnetic manipulation and spatial patterning of multi-cellular stem cell aggregates
- PMID: 22076329
- PMCID: PMC4633527
- DOI: 10.1039/c1ib00064k
Magnetic manipulation and spatial patterning of multi-cellular stem cell aggregates
Abstract
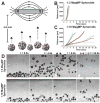
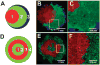
The controlled assembly and organization of multi-cellular systems to mimic complex tissue structures is critical to the engineering of tissues for therapeutic and diagnostic applications. Recent advances in micro-scale technologies to control multi-cellular aggregate formation typically require chemical modification of the interface between cells and materials and lack multi-scale flexibility. Here we demonstrate that simple physical entrapment of magnetic microparticles within the extracellular space of stem cells spheroids during initial formation enables scaffold-free immobilization, translocation and directed assembly of multi-cellular aggregates across multiple length and time scales, even under dynamic suspension culture conditions. The response of aggregates to externally applied magnetic fields was a direct function of microparticle incorporation, allowing for rapid and transient control of the extracellular environment as well as separation of heterogeneous populations. In addition, spatial patterning of heterogeneous spheroid populations as well as individual multi-cellular aggregates was readily achieved by imposing temporary magnetic fields. Overall, this approach provides novel routes to examine stem cell differentiation and tissue morphogenesis with applications that encompass the creation of new model systems for developmental biology, scaffold-free tissue engineering strategies and scalable bioprocessing technologies.
Figures



References
-
- Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–57. - PubMed
-
- Khetani SR, Bhatia SN. Microscale culture of human liver cells for drug development. Nat Biotechnol. 2008;26:120–6. - PubMed
-
- Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6. - PubMed
-
- Takeichi M. Cadherins: a molecular family important in selective cell-cell adhesion. Annu Rev Biochem. 1990;59:237–52. - PubMed
-
- Hynes RO, Lander AD. Contact and adhesive specificities in the associations, migrations, and targeting of cells and axons. Cell. 1992;68:303–22. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

