N₂reduction and hydrogenation to ammonia by a molecular iron-potassium complex
- PMID: 22076372
- PMCID: PMC3218428
- DOI: 10.1126/science.1211906
N₂reduction and hydrogenation to ammonia by a molecular iron-potassium complex
Erratum in
- Science. 2014 Feb 21;343(6173):839
Abstract
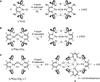
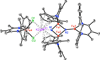
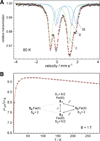
The most common catalyst in the Haber-Bosch process for the hydrogenation of dinitrogen (N(2)) to ammonia (NH(3)) is an iron surface promoted with potassium cations (K(+)), but soluble iron complexes have neither reduced the N-N bond of N(2) to nitride (N(3-)) nor produced large amounts of NH(3) from N(2). We report a molecular iron complex that reacts with N(2) and a potassium reductant to give a complex with two nitrides, which are bound to iron and potassium cations. The product has a Fe(3)N(2) core, implying that three iron atoms cooperate to break the N-N triple bond through a six-electron reduction. The nitride complex reacts with acid and with H(2) to give substantial yields of N(2)-derived ammonia. These reactions, although not yet catalytic, give structural and spectroscopic insight into N(2) cleavage and N-H bond-forming reactions of iron.
Figures

References
-
- Smil V. Sci. Am. 1997;277:76.
-
- Mittasch A. Geschichte der Ammoniaksynthese. Weinheim: Verlag Chemie; 1951.
-
- Schlögl R. Handbook of Heterogeneous Catalysis. 2nd Ed. vol. 5. Weinheim: Wiley-VCH; 2008. pp. 2501–2575.
-
- Hellman A, et al. J. Phys. Chem. B. 2006;110:17719. - PubMed
-
- Burgess BK, Lowe DJ. Chem. Rev. 1996;96:2983. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

