High mobility group box protein 1 (HMGB1)-partner molecule complexes enhance cytokine production by signaling through the partner molecule receptor
- PMID: 22076468
- PMCID: PMC3320135
- DOI: 10.2119/molmed.2011.00327
High mobility group box protein 1 (HMGB1)-partner molecule complexes enhance cytokine production by signaling through the partner molecule receptor
Abstract
The nuclear protein high mobility group box protein 1 (HMGB1) promotes inflammation upon extracellular release. HMGB1 induces proinflammatory cytokine production in macrophages via Toll-like receptor (TLR)-4 signaling in a redox-dependent fashion. Independent of its redox state and endogenous cytokine-inducing ability, HMGB1 can form highly immunostimulatory complexes by interaction with certain proinflammatory mediators. Such complexes have the ability to enhance the induced immune response up to 100-fold, compared with induction by the ligand alone. To clarify the mechanisms for these strong synergistic effects, we studied receptor requirements. Interleukin (IL)-6 production was assessed in supernatants from cultured peritoneal macrophages from mice each deficient in one of the HMGB1 receptors (receptor for advanced glycation end products [RAGE], TLR2 or TLR4) or from wild-type controls. The cultures were stimulated with the TLR4 ligand lipopolysaccaride (LPS), the TLR2 ligand Pam₃CysSerLys₄ (Pam₃CSK₄), noninflammatory HMGB1 or each TLR ligand in complex with noninflammatory HMGB1. The activity of the HMGB1-TLR ligand complexes relied on engagement of the same receptor as for the noncomplexed TLR ligand, since HMGB1-LPS complexes used TLR4 and HMGB1-Pam₃CSK₄ complexes used TLR2. Deletion of any of the intracellular adaptor molecules used by TLR2 (myeloid differentiation factor-88 [MyD88], TIR domain-containing adaptor protein [TIRAP]) or TLR4 (MyD88, TIRAP, TIR domain-containing adaptor-inducing interferon-β [TRIF], TRIF-related adaptor molecule [TRAM]) had similar effects on HMGB1 complex activation compared with noncomplexed LPS or Pam₃CSK₄. This result implies that the enhancing effects of HMGB1-partner molecule complexes are not regulated by the induction of additional signaling cascades. Elucidating HMGB1 receptor usage in processes where HMGB1 acts alone or in complex with other molecules is essential for the understanding of basic HMGB1 biology and for designing HMGB1-targeted therapies.
Figures
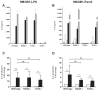
 , HMGB1 (1 μg/mL);■, HMGB1 (1 μg/mL) + LPS (10 ng/mL);
, HMGB1 (1 μg/mL);■, HMGB1 (1 μg/mL) + LPS (10 ng/mL);
 , LPS (1 μg/mL); (B, D) ▨, unstimulated;□, Pam3CSK4 (10 ng/mL);
, LPS (1 μg/mL); (B, D) ▨, unstimulated;□, Pam3CSK4 (10 ng/mL);
 , HMGB1 (20 μg/mL);■, HMGB1 (20 μg/mL) + Pam3CSK4 (10 ng/mL);
, HMGB1 (20 μg/mL);■, HMGB1 (20 μg/mL) + Pam3CSK4 (10 ng/mL);
 , Pam3CSK4 (10 μg/mL).
, Pam3CSK4 (10 μg/mL).
 , HMGB1 (1 μg/mL);■, HMGB1 (1 μg/mL) + LPS (1 ng/mL);
, HMGB1 (1 μg/mL);■, HMGB1 (1 μg/mL) + LPS (1 ng/mL);
 , LPS (1 μg/mL); (B) ▨, unstimulated;□, Pam3CSK4 (10 ng/mL);
, LPS (1 μg/mL); (B) ▨, unstimulated;□, Pam3CSK4 (10 ng/mL);
 , HMGB1 (20 μg/mL);■, HMGB1 (20 μg/mL) + Pam3CSK4 (10 ng/mL);
, HMGB1 (20 μg/mL);■, HMGB1 (20 μg/mL) + Pam3CSK4 (10 ng/mL);
 , Pam3CSK4 (10 μg/mL).
, Pam3CSK4 (10 μg/mL).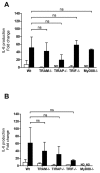
References
-
- Wang H, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–51. - PubMed
-
- Semino C, Angelini G, Poggi A, Rubartelli A. NK/iDC interaction results in IL-18 secretion by DCs at the synaptic cleft followed by NK cell activation and release of the DC maturation factor HMGB1. Blood. 2005;106:609–16. - PubMed
-
- Dumitriu IE, et al. Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J Immunol. 2005;174:7506–15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

