Intrathecal effects of daclizumab treatment of multiple sclerosis
- PMID: 22076546
- PMCID: PMC3246406
- DOI: 10.1212/WNL.0b013e318239f7ef
Intrathecal effects of daclizumab treatment of multiple sclerosis
Abstract
Objectives: We previously reported that daclizumab, a humanized monoclonal antibody against CD25, reduced contrast-enhancing lesions (CEL) in patients with multiple sclerosis (MS) who were suboptimal responders to interferon-β and that this response correlated with expansion of CD56(bright) NK cells. These data have been reproduced in a placebo-controlled multicenter trial (CHOICE study). The current study investigates whether daclizumab monotherapy reduces CEL in untreated patients with relapsing-remitting MS (RRMS) and the effects of daclizumab on the intrathecal immune system.
Methods: Sixteen patients with RRMS with high inflammatory activity were enrolled in an open-label, baseline-vs-treatment, phase II trial of daclizumab monotherapy for 54 weeks and followed by serial clinical and MRI examinations and immunologic biomarkers measured in the whole blood and CSF.
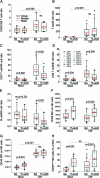
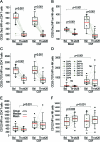
Results: The trial achieved predefined outcomes. There was an 87.7% reduction in brain CEL (primary) and improvements in Multiple Sclerosis Functional Composite (secondary), Scripps Neurologic Rating Scale, and Expanded Disability Status Scale (tertiary) outcomes. There was significant expansion of CD56(bright) NK cells in peripheral blood and CSF, with resultant decrease in T cells/NK cells and B cells/NK cells ratios and IL-12p40 in the CSF. Surprisingly, CD25 Tac epitope was equally blocked on the immune cells in the CSF and in peripheral blood.
Conclusions: Daclizumab monotherapy inhibits formation of MS plaques in patients with RRMS and immunoregulatory NK cells may suppress activation of pathogenic immune responses directly in the CNS compartment.
Classification of evidence: The study provides Class III evidence that daclizumab reduces the number of contrast-enhancing lesions in treatment-naive patients with RRMS over a 54-week period.
Figures



References
-
- Rose JW, Burns JB, Bjorklund J, Klein J, Watt HE, Carlson NG. Daclizumab phase II trial in relapsing and remitting multiple sclerosis: MRI and clinical results. Neurology 2007;69:785–789 - PubMed
-
- Wynn D, Kaufman M, Montalban X, et al. Daclizumab in active relapsing multiple sclerosis (CHOICE study): a phase 2, randomised, double-blind, placebo-controlled, add-on trial with interferon beta. Lancet Neurol 2010;9:381–390 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
