Exenatide as a weight-loss therapy in extreme pediatric obesity: a randomized, controlled pilot study
- PMID: 22076596
- PMCID: PMC3684414
- DOI: 10.1038/oby.2011.337
Exenatide as a weight-loss therapy in extreme pediatric obesity: a randomized, controlled pilot study
Abstract
The objective of this pilot study was to evaluate the effects of exenatide on BMI (primary endpoint) and cardiometabolic risk factors in nondiabetic youth with extreme obesity. Twelve children and adolescents (age 9-16 years old) with extreme obesity (BMI ≥1.2 times the 95th percentile or BMI ≥35 kg/m(2)) were enrolled in a 6-month, randomized, open-label, crossover, clinical trial consisting of two, 3-month phases: (i) a control phase of lifestyle modification and (ii) a drug phase of lifestyle modification plus exenatide. Participants were equally randomized to phase-order (i.e., starting with control or drug therapy) then crossed-over to the other treatment. BMI, body fat percentage, blood pressure, lipids, oral glucose tolerance tests (OGTT), adipokines, plasma biomarkers of endothelial activation, and endothelial function were assessed at baseline, 3-, and 6-months. The mean change over each 3-month phase was compared between treatments. Compared to control, exenatide significantly reduced BMI (-1.7 kg/m(2), 95% confidence interval (CI) (-3.0, -0.4), P = 0.01), body weight (-3.9 kg, 95% CI (-7.11, -0.69), P = 0.02), and fasting insulin (-7.5 mU/l, 95% CI (-13.71, -1.37), P = 0.02). Significant improvements were observed for OGTT-derived insulin sensitivity (P = 0.02) and β-cell function (P = 0.03). Compliance with the injection regimen was excellent (≥94%) and exenatide was generally well-tolerated (the most common adverse event was mild nausea in 36%). These preliminary data suggest that exenatide should be evaluated in larger, well-controlled trials for its ability to reduce BMI and improve cardiometabolic risk factors in youth with extreme obesity.
Trial registration: ClinicalTrials.gov NCT00886626.
Figures

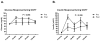
References
-
- Flegal KM, Wei R, Ogden CL, Freedman DS, Johnson CL, Curtin LR. Characterizing extreme values of body mass index-for-age by using the 2000 Centers for Disease Control and Prevention growth charts. Am J Clin Nutr. 2009 Nov;90(5):1314–20. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

