Integration of repulsive guidance cues generates avascular zones that shape mammalian blood vessels
- PMID: 22076636
- PMCID: PMC3253216
- DOI: 10.1161/CIRCRESAHA.111.249847
Integration of repulsive guidance cues generates avascular zones that shape mammalian blood vessels
Abstract
Rationale: Positive signals, such as vascular endothelial growth factor, direct endothelial cells (ECs) to specific locations during blood vessel formation. Less is known about repulsive signal contribution to shaping vessels. Recently, "neuronal guidance cues" have been shown to influence EC behavior, particularly in directing sprouting angiogenesis by repelling ECs. However, their role during de novo blood vessel formation remains unexplored.
Objective: To identify signals that guide and pattern the first mammalian blood vessels.
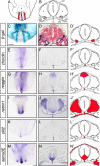
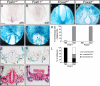
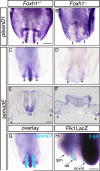
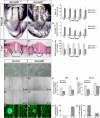
Methods and results: Using genetic mouse models, we show that blood vessels are sculpted through the generation of stereotyped avascular zones by EC-repulsive cues. We demonstrate that Semaphorin3E (Sema3E) is a key factor that shapes the paired dorsal aortae in mouse, as sema3E(-/-) embryos develop an abnormally branched aortic plexus with a markedly narrowed avascular midline. In vitro cultures and avian grafting experiments show strong repulsion of ECs by Sema3E-expressing cells. We further identify the mouse notochord as a rich source of multiple redundant neuronal guidance cues. Mouse embryos that lack notochords fail to form cohesive aortic vessels because of loss of the avascular midline, yet maintain lateral avascular zones. We demonstrate that lateral avascular zones are directly generated by the lateral plate mesoderm, a critical source of Sema3E.
Conclusions: These findings demonstrate that Sema3E-generated avascular zones are critical regulators of mammalian cardiovascular patterning and are the first to identify a repulsive role for the lateral plate mesoderm. Integration of multiple, and in some cases redundant, repulsive cues from various tissues is critical to patterning the first embryonic blood vessels.
Figures


Similar articles
-
Resolution of defective dorsal aortae patterning in Sema3E-deficient mice occurs via angiogenic remodeling.Dev Dyn. 2013 May;242(5):580-90. doi: 10.1002/dvdy.23949. Epub 2013 Mar 29. Dev Dyn. 2013. PMID: 23444297 Free PMC article.
-
Antagonistic interactions among Plexins regulate the timing of intersegmental vessel formation.Dev Biol. 2009 Jul 15;331(2):199-209. doi: 10.1016/j.ydbio.2009.04.037. Epub 2009 May 5. Dev Biol. 2009. PMID: 19422817
-
Negative regulation of midline vascular development by the notochord.Dev Cell. 2004 May;6(5):699-708. doi: 10.1016/s1534-5807(04)00127-3. Dev Cell. 2004. PMID: 15130494
-
Vascular patterning: coordinated signals keep blood vessels on track.Curr Opin Genet Dev. 2015 Jun;32:86-91. doi: 10.1016/j.gde.2015.02.002. Epub 2015 Mar 3. Curr Opin Genet Dev. 2015. PMID: 25748252 Review.
-
VEGF-directed blood vessel patterning: from cells to organism.Cold Spring Harb Perspect Med. 2012 Sep 1;2(9):a006452. doi: 10.1101/cshperspect.a006452. Cold Spring Harb Perspect Med. 2012. PMID: 22951440 Free PMC article. Review.
Cited by
-
Spatiotemporal Characterization of Human Early Intervertebral Disc Formation at Single-Cell Resolution.Adv Sci (Weinh). 2023 May;10(14):e2206296. doi: 10.1002/advs.202206296. Epub 2023 Mar 25. Adv Sci (Weinh). 2023. PMID: 36965031 Free PMC article.
-
Navigation rules for vessels and neurons: cooperative signaling between VEGF and neural guidance cues.Cell Mol Life Sci. 2013 May;70(10):1685-703. doi: 10.1007/s00018-013-1278-4. Epub 2013 Mar 12. Cell Mol Life Sci. 2013. PMID: 23475066 Free PMC article. Review.
-
Role of Semaphorin Signaling During Cardiovascular Development.J Am Heart Assoc. 2018 May 18;7(11):e008853. doi: 10.1161/JAHA.118.008853. J Am Heart Assoc. 2018. PMID: 29776958 Free PMC article. Review. No abstract available.
-
Characterization of Semaphorin 6A-Mediated Effects on Angiogenesis Through Regulation of VEGF Signaling.Methods Mol Biol. 2017;1493:345-361. doi: 10.1007/978-1-4939-6448-2_25. Methods Mol Biol. 2017. PMID: 27787863 Free PMC article.
-
Endothelial PlexinD1 signaling instructs spinal cord vascularization and motor neuron development.Neuron. 2022 Dec 21;110(24):4074-4089.e6. doi: 10.1016/j.neuron.2022.12.005. Neuron. 2022. PMID: 36549270 Free PMC article.
References
-
- Mukouyama YS, Shin D, Britsch S, Taniguchi M, Anderson DJ. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002;109(6):693–705. - PubMed
-
- Hogan KA, Ambler CA, Chapman DL, Bautch VL. The neural tube patterns vessels developmentally using the VEGF signaling pathway. Development. 2004;131(7):1503–1513. - PubMed
-
- Eichmann A, Makinen T, Alitalo K. Neural guidance molecules regulate vascular remodeling and vessel navigation. Genes Dev. 2005;19(9):1013–1021. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

