Transfusion in the absence of inflammation induces antigen-specific tolerance to murine RBCs
- PMID: 22077064
- PMCID: PMC3286217
- DOI: 10.1182/blood-2011-09-382655
Transfusion in the absence of inflammation induces antigen-specific tolerance to murine RBCs
Erratum in
- Blood. 2014 Mar 27;123(13):1182
Abstract
Most human transfusion recipients fail to make detectable alloantibodies to foreign RBC antigens ("nonresponders"). Herein, we use a murine model to test the hypothesis that nonresponders may be immunologically tolerant. FVB mice transfused with RBCs expressing transgenic human glycophorin A (hGPA) antigen in the absence of inflammation produced undetectable levels of anti-hGPA immunoglobulins, unlike those transfused in the presence of polyinosinic:polycytidylic acid-induced inflammation. Mice in the nonresponder group failed to produce anti-hGPA after subsequent transfusions in the presence of polyinosinic:polycytidylic acid, whereas anti-hGPA levels increased in the responder group. This tolerance was antigen specific, because nonresponders to hGPA produced alloantibodies to RBCs that expressed a different transgenic antigen. This tolerance was not an idiosyncrasy of the hGPA antigen nor of the recipient strain, because B10.BR mice transfused with membrane-bound hen egg lysozyme antigen-transgenic RBCs also demonstrated induced nonresponsiveness. These data demonstrate that RBCs transfused in the absence of inflammation can induce tolerance.
Figures
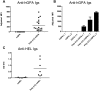
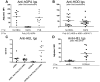
Comment in
-
Tolerant heaven or mHEL trouble.Blood. 2012 Feb 9;119(6):1330-1. doi: 10.1182/blood-2011-11-393470. Blood. 2012. PMID: 22323408
References
-
- Seyfried H, Walewska I. Analysis of immune response to red blood cell antigens in multitransfused patients with different diseases. Mater Med Pol. 1990;22(1):21–25. - PubMed
-
- Tormey CA, Fisk J, Stack G. Red blood cell alloantibody frequency, specificity, and properties in a population of male military veterans. Transfusion. 2008;48(10):2069–2076. - PubMed
-
- Noizat-Pirenne F, Tournamille C, Bierling P, et al. Relative immunogenicity of Fya and K antigens in a caucasian population, based on HLA class II restriction analysis. Transfusion. 2006;46(8):1328–1333. - PubMed
-
- Frohn C, Dumbgen L, Brand JM, Gorg S, Luhm J, Kirchner H. Probability of anti-D development in D− patients receiving D+ RBCs. Transfusion. 2003;43(7):893–898. - PubMed
-
- Yazer MH, Triulzi DJ. Detection of anti-D in D− recipients transfused with D+ red blood cells. Transfusion. 2007;47(12):2197–2201. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

