Stop the flow: a paradigm for cell signaling mediated by reactive oxygen species in the pulmonary endothelium
- PMID: 22077215
- PMCID: PMC4476273
- DOI: 10.1146/annurev-physiol-020911-153324
Stop the flow: a paradigm for cell signaling mediated by reactive oxygen species in the pulmonary endothelium
Abstract
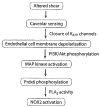
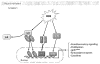
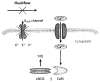
The lung endothelium is exposed to mechanical stimuli through shear stress arising from blood flow and responds to altered shear by activation of NADPH (NOX2) to generate reactive oxygen species (ROS). This review describes the pathway for NOX2 activation and the downstream ROS-mediated signaling events on the basis of studies of isolated lungs and flow-adapted endothelial cells in vitro that are subjected to acute flow cessation (ischemia). Altered mechanical stress is detected by a cell-associated complex involving caveolae and other membrane proteins that results in endothelial cell membrane depolarization and then the activation of specific kinases that lead to the assembly of NOX2 components. ROS generated by this enzyme amplify the mechanosignal within the endothelial cell to regulate activation and/or synthesis of proteins that participate in cell growth, proliferation, differentiation, apoptosis, and vascular remodeling. These responses indicate an important role for NOX2-derived ROS associated with mechanotransduction in promoting vascular homeostasis.
Figures

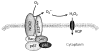


Similar articles
-
Shear stress-related mechanosignaling with lung ischemia: lessons from basic research can inform lung transplantation.Am J Physiol Lung Cell Mol Physiol. 2014 Nov 1;307(9):L668-80. doi: 10.1152/ajplung.00198.2014. Epub 2014 Sep 19. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 25239915 Free PMC article. Review.
-
PECAM-1 and caveolae form the mechanosensing complex necessary for NOX2 activation and angiogenic signaling with stopped flow in pulmonary endothelium.Am J Physiol Lung Cell Mol Physiol. 2013 Dec;305(11):L805-18. doi: 10.1152/ajplung.00123.2013. Epub 2013 Sep 27. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 24077950 Free PMC article.
-
Lung endothelial cell proliferation with decreased shear stress is mediated by reactive oxygen species.Am J Physiol Cell Physiol. 2006 Jan;290(1):C66-76. doi: 10.1152/ajpcell.00094.2005. Epub 2005 Aug 17. Am J Physiol Cell Physiol. 2006. PMID: 16107509
-
Membrane depolarization is the trigger for PI3K/Akt activation and leads to the generation of ROS.Am J Physiol Heart Circ Physiol. 2012 Jan 1;302(1):H105-14. doi: 10.1152/ajpheart.00298.2011. Epub 2011 Oct 14. Am J Physiol Heart Circ Physiol. 2012. PMID: 22003059 Free PMC article.
-
Lung ischemia: a model for endothelial mechanotransduction.Cell Biochem Biophys. 2008;52(3):125-38. doi: 10.1007/s12013-008-9030-7. Epub 2008 Nov 4. Cell Biochem Biophys. 2008. PMID: 18982455 Free PMC article. Review.
Cited by
-
Peroxiredoxin6 in Endothelial Signaling.Antioxidants (Basel). 2019 Mar 13;8(3):63. doi: 10.3390/antiox8030063. Antioxidants (Basel). 2019. PMID: 30871234 Free PMC article. Review.
-
Tipping the balance from angiogenesis to fibrosis in CKD.Kidney Int Suppl (2011). 2014 Nov;4(1):45-52. doi: 10.1038/kisup.2014.9. Kidney Int Suppl (2011). 2014. PMID: 26312149 Free PMC article. Review.
-
Microtubules tune mechanotransduction through NOX2 and TRPV4 to decrease sclerostin abundance in osteocytes.Sci Signal. 2017 Nov 21;10(506):eaan5748. doi: 10.1126/scisignal.aan5748. Sci Signal. 2017. PMID: 29162742 Free PMC article.
-
Hemodynamic regulation of reactive oxygen species: implications for vascular diseases.Antioxid Redox Signal. 2014 Feb 20;20(6):914-28. doi: 10.1089/ars.2013.5507. Epub 2013 Sep 17. Antioxid Redox Signal. 2014. PMID: 23879326 Free PMC article. Review.
-
Comparative analysis of the mechanical signals in lung development and compensatory growth.Cell Tissue Res. 2017 Mar;367(3):687-705. doi: 10.1007/s00441-016-2558-8. Epub 2017 Jan 13. Cell Tissue Res. 2017. PMID: 28084523 Free PMC article. Review.
References
-
- Forman HJ, Torres M, Fukuto J. Redox signaling. Mol Cell Biochem. 2002;234/235:49–62. - PubMed
-
- Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–89. - PubMed
-
- Davies PF, Barbee KA, Volin MV, Robotewskyj A, Chen J, et al. Spatial relationships in early signaling events of flow-mediated endothelial mechanotransduction. Annu Rev Physiol. 1997;59:527–49. - PubMed
-
- Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

