Pathogenesis of plexiform neurofibroma: tumor-stromal/hematopoietic interactions in tumor progression
- PMID: 22077553
- PMCID: PMC3694738
- DOI: 10.1146/annurev-pathol-011811-132441
Pathogenesis of plexiform neurofibroma: tumor-stromal/hematopoietic interactions in tumor progression
Abstract
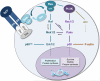
Neurofibromatosis type 1 (NF1) is a genetic disease that results from either heritable or spontaneous autosomal dominant mutations in the NF1 gene. A second-hit mutation precedes the predominant NF1 neoplasms, which include myeloid leukemia, optic glioma, and plexiform neurofibroma. Despite this requisite NF1 loss of heterozygosity in the tumor cell of origin, nontumorigenic cells contribute to both generalized and specific disease manifestations. In mouse models of plexiform neurofibroma formation, Nf1 haploinsufficient mast cells promote inflammation, accelerating tumor formation and growth. These recruited mast cells, hematopoietic effector cells long known to permeate neurofibroma tissue, mediate key mitogenic signals that contribute to vascular ingrowth, collagen deposition, and tumor growth. Thus, the plexiform neurofibroma microenvironment involves a tumor/stromal interaction with the hematopoietic system that depends, at the molecular level, on a stem cell factor/c-kit-mediated signaling axis. These observations parallel findings in other NF1 disease manifestations and are clearly relevant to medical management of these neurofibromas.
Figures



References
-
- Friedman J, Gutmann DH, Maccollin M, Riccardi VM. Neurofibromatosis: phenotype, natural history, and pathogenesis. The Johns Hopkins University Press; Baltimore: 1999. p. 381.
-
- Lubs ML, Bauer MS, Formas ME, Djokic B. Lisch nodules in neurofibromatosis type 1. N Engl J Med. 1991;324:1264–6. - PubMed
-
- Riccardi VM. Neurofibromatosis: past, present, and future. N Engl J Med. 1991;324:1283–5. - PubMed
-
- Riccardi VM. Neurofibromatosis type 1 is a disorder of dysplasia: the importance of distinguishing features, consequences, and complications. Birth Defects Res A Clin Mol Teratol. 2010;88:9–14. - PubMed
-
- Akenside M. Observations on cancers. Med Trans Coll Phys Lond. 1768;1:64–92.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

