Long-term expression of human coagulation factor VIII in a tolerant mouse model using the φC31 integrase system
- PMID: 22077817
- PMCID: PMC3327602
- DOI: 10.1089/hum.2011.110
Long-term expression of human coagulation factor VIII in a tolerant mouse model using the φC31 integrase system
Abstract
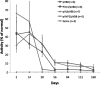
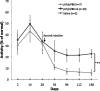
We generated a mouse model for hemophilia A that combines a homozygous knockout for murine factor VIII (FVIII) and a homozygous addition of a mutant human FVIII (hFVIII). The resulting mouse, having no detectable FVIII protein or activity and tolerant to hFVIII, is useful for evaluating FVIII gene-therapy protocols. This model was used to develop an effective gene-therapy strategy using the φC31 integrase to mediate permanent genomic integration of an hFVIII cDNA deleted for the B-domain. Various plasmids encoding φC31 integrase and hFVIII were delivered to the livers of these mice by using hydrodynamic tail-vein injection. Long-term expression of therapeutic levels of hFVIII was observed over a 6-month time course when an intron was included in the hFVIII expression cassette and wild-type φC31 integrase was used. A second dose of the hFVIII and integrase plasmids resulted in higher long-term hFVIII levels, indicating that incremental doses were beneficial and that a second dose of φC31 integrase was tolerated. We observed a significant decrease in the bleeding time after a tail-clip challenge in mice treated with plasmids expressing hFVIII and φC31 integrase. Genomic integration of the hFVIII expression plasmid was demonstrated by junction PCR at a known hotspot for integration in mouse liver. The φC31 integrase system provided a nonviral method to achieve long-term FVIII gene therapy in a relevant mouse model of hemophilia A.
Figures




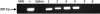
References
-
- Bi L. Lawler A.M. Antonarakis S.E., et al. Targeted disruption of the mouse factor VIII gene produces a model of haemophilia A. Nat. Genet. 1995;10:119–121. - PubMed
-
- Brettler D.B. Kraus E.M. Levine P.H. Clinical Aspects of and Therapy for Hemophilia A. Churchill Livingstone; New York, NY: 1995. pp. 1648–1663.
-
- Bril W.S. MacLean P.E. Kaijen P.H., et al. HLA class II genotype and factor VIII inhibitors in mild hemophilia A patients with Arg593 to Cys mutation. Haemophilia. 2004;5:509–514. - PubMed
-
- Bril W.S. van Helden P.M. Hausl C., et al. Tolerance to factor VIII in a transgenic mouse expressing human factor VIII cDNA carrying an Arg(593) to Cys substitution. Thromb. Haemost. 2006;95:341–347. - PubMed
-
- Chalberg T.C. Portlock J.L. Olivares E.C., et al. Integration specificity of phage phiC31 integrase in the human genome. J. Mol. Biol. 2006;357:28–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

