Muscarinic Acetylcholine Receptor M3 Mutation Causes Urinary Bladder Disease and a Prune-Belly-like Syndrome
- PMID: 22077972
- PMCID: PMC3213389
- DOI: 10.1016/j.ajhg.2011.10.007
Muscarinic Acetylcholine Receptor M3 Mutation Causes Urinary Bladder Disease and a Prune-Belly-like Syndrome
Abstract
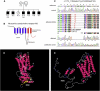
Urinary bladder malformations associated with bladder outlet obstruction are a frequent cause of progressive renal failure in children. We here describe a muscarinic acetylcholine receptor M3 (CHRM3) (1q41-q44) homozygous frameshift mutation in familial congenital bladder malformation associated with a prune-belly-like syndrome, defining an isolated gene defect underlying this sometimes devastating disease. CHRM3 encodes the M3 muscarinic acetylcholine receptor, which we show is present in developing renal epithelia and bladder muscle. These observations may imply that M3 has a role beyond its known contribution to detrusor contractions. This Mendelian disease caused by a muscarinic acetylcholine receptor mutation strikingly phenocopies Chrm3 null mutant mice.
Copyright © 2011 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) (2010). Annual Transplant Report. https://web.emmes.com/study/ped/annlrept/2010_Report.pdf.
-
- Weber S., Moriniere V., Knüppel T., Charbit M., Dusek J., Ghiggeri G.M., Jankauskiené A., Mir S., Montini G., Peco-Antic A., et al. Prevalence of mutations in renal developmental genes in children with renal hypodysplasia: results of the ESCAPE study. J. Am. Soc. Nephrol. 2006;17:2864–2870. - PubMed
-
- Farrugia M.-K., Woolf A.S. Congenital urinary bladder outlet obstruction. Fetal Matern. Med. Rev. 2010;21:55–73.
-
- Weber S., Mir S., Schlingmann K.P., Nürnberg G., Becker C., Kara P.E., Ozkayin N., Konrad M., Nürnberg P., Schaefer F. Gene locus ambiguity in posterior urethral valves/prune-belly syndrome. Pediatr. Nephrol. 2005;20:1036–1042. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

