Role and expression of FRS2 and FRS3 in prostate cancer
- PMID: 22078327
- PMCID: PMC3231952
- DOI: 10.1186/1471-2407-11-484
Role and expression of FRS2 and FRS3 in prostate cancer
Abstract
Background: FGF receptor substrates (FRS2 and FRS3) are key adaptor proteins that mediate FGF-FGFR signalling in benign as well as malignant tissue. Here we investigated FRS2 and FRS3 as a means of disrupting global FGF signalling in prostate cancer.
Methods: FRS2 and FRS3 manipulation was investigated in vitro using over-expression, knockdown and functional assays. FRS2 and FRS3 expression was profiled in cell lines and clinical tumors of different grades.
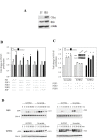
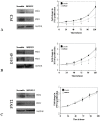
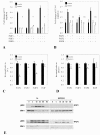
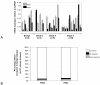
Results: In a panel of cell lines we observed ubiquitous FRS2 and FRS3 transcript and protein expression in both benign and malignant cells. We next tested functional redundancy of FRS2 and FRS3 in prostate cancer cells. In DU145 cells, specific FRS2 suppression inhibited FGF induced signalling. This effect was not apparent in cells stably over-expressing FRS3. Indeed FRS3 over-expression resulted in enhanced proliferation (p = 0.005) compared to control cells. Given this functional redundancy, we tested the therapeutic principle of dual targeting of FRS2 and FRS3 in prostate cancer. Co-suppression of FRS2 and FRS3 significantly inhibited ERK activation with a concomitant reduction in cell proliferation (p < 0.05), migration and invasion (p < 0.05). Synchronous knockdown of FRS2 and FRS3 with exposure to cytotoxic irradiation resulted in a significant reduction in prostate cancer cell survival compared to irradiation alone (p < 0.05). Importantly, this synergistic effect was not observed in benign cells. Finally, we investigated expression of FRS2 and FRS3 transcript in a cohort of micro-dissected tumors of different grades as well as by immunohistochemistry in clinical biopsies. Here, we did not observe any difference in expression between benign and malignant biopsies.
Conclusions: These results suggest functional overlap of FRS2 and FRS3 in mediating mitogenic FGF signalling in the prostate. FRS2 and FRS3 are not over-expressed in tumours but targeted dual inhibition may selectively adversely affect malignant but not benign prostate cells.
Figures



Similar articles
-
The fibroblast growth factor receptor substrate 3 adapter is a developmentally regulated microtubule-associated protein expressed in migrating and differentiated neurons.J Neurochem. 2010 Feb;112(4):924-39. doi: 10.1111/j.1471-4159.2009.06503.x. Epub 2009 Nov 24. J Neurochem. 2010. PMID: 19943849
-
Amplification of FRS2 and activation of FGFR/FRS2 signaling pathway in high-grade liposarcoma.Cancer Res. 2013 Feb 15;73(4):1298-307. doi: 10.1158/0008-5472.CAN-12-2086. Epub 2013 Feb 7. Cancer Res. 2013. PMID: 23393200
-
Genomic organization and comparative sequence analysis of the mouse and human FRS2, FRS3 genes.Mol Biol Rep. 2003 Mar;30(1):15-25. doi: 10.1023/a:1022231426309. Mol Biol Rep. 2003. PMID: 12688531
-
Pharmacologically targeting the myristoylation of the scaffold protein FRS2α inhibits FGF/FGFR-mediated oncogenic signaling and tumor progression.J Biol Chem. 2018 Apr 27;293(17):6434-6448. doi: 10.1074/jbc.RA117.000940. Epub 2018 Mar 14. J Biol Chem. 2018. PMID: 29540482 Free PMC article.
-
Evidence for downregulation of the negative regulator SPRED2 in clinical prostate cancer.Br J Cancer. 2013 Feb 19;108(3):597-601. doi: 10.1038/bjc.2012.507. Epub 2012 Nov 20. Br J Cancer. 2013. PMID: 23169297 Free PMC article.
Cited by
-
Proteogenomic analysis reveals exosomes are more oncogenic than ectosomes.Oncotarget. 2015 Jun 20;6(17):15375-96. doi: 10.18632/oncotarget.3801. Oncotarget. 2015. PMID: 25944692 Free PMC article.
-
MiR-4653-3p and its target gene FRS2 are prognostic biomarkers for hormone receptor positive breast cancer patients receiving tamoxifen as adjuvant endocrine therapy.Oncotarget. 2016 Sep 20;7(38):61166-61182. doi: 10.18632/oncotarget.11278. Oncotarget. 2016. PMID: 27533459 Free PMC article.
-
Targeted genomic analysis of 364 adrenocortical carcinomas.Endocr Relat Cancer. 2021 Aug 16;28(10):671-681. doi: 10.1530/ERC-21-0040. Endocr Relat Cancer. 2021. PMID: 34410225 Free PMC article.
-
Identification of miRNA and genes involving in osteosarcoma by comprehensive analysis of microRNA and copy number variation data.Oncol Lett. 2017 Nov;14(5):5427-5433. doi: 10.3892/ol.2017.6845. Epub 2017 Aug 28. Oncol Lett. 2017. PMID: 29098032 Free PMC article.
-
FGFs: crucial factors that regulate tumour initiation and progression.Cell Prolif. 2016 Aug;49(4):438-47. doi: 10.1111/cpr.12275. Epub 2016 Jul 7. Cell Prolif. 2016. PMID: 27383016 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

