CD14 controls the LPS-induced endocytosis of Toll-like receptor 4
- PMID: 22078883
- PMCID: PMC3217211
- DOI: 10.1016/j.cell.2011.09.051
CD14 controls the LPS-induced endocytosis of Toll-like receptor 4
Abstract
The transport of Toll-like Receptors (TLRs) to various organelles has emerged as an essential means by which innate immunity is regulated. While most of our knowledge is restricted to regulators that promote the transport of newly synthesized receptors, the regulators that control TLR transport after microbial detection remain unknown. Here, we report that the plasma membrane localized Pattern Recognition Receptor (PRR) CD14 is required for the microbe-induced endocytosis of TLR4. In dendritic cells, this CD14-dependent endocytosis pathway is upregulated upon exposure to inflammatory mediators. We identify the tyrosine kinase Syk and its downstream effector PLCγ2 as important regulators of TLR4 endocytosis and signaling. These data establish that upon microbial detection, an upstream PRR (CD14) controls the trafficking and signaling functions of a downstream PRR (TLR4). This innate immune trafficking cascade illustrates how pathogen detection systems operate to induce both membrane transport and signal transduction.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


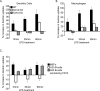


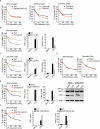
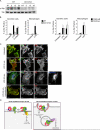
References
-
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

