A mechanism for the evolution of phosphorylation sites
- PMID: 22078888
- PMCID: PMC3220604
- DOI: 10.1016/j.cell.2011.08.052
A mechanism for the evolution of phosphorylation sites
Abstract
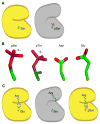
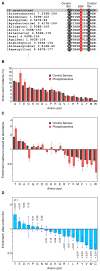
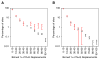
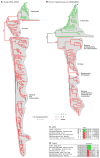
Protein phosphorylation provides a mechanism for the rapid, reversible control of protein function. Phosphorylation adds negative charge to amino acid side chains, and negatively charged amino acids (Asp/Glu) can sometimes mimic the phosphorylated state of a protein. Using a comparative genomics approach, we show that nature also employs this trick in reverse by evolving serine, threonine, and tyrosine phosphorylation sites from Asp/Glu residues. Structures of three proteins where phosphosites evolved from acidic residues (DNA topoisomerase II, enolase, and C-Raf) show that the relevant acidic residues are present in salt bridges with conserved basic residues, and that phosphorylation has the potential to conditionally restore the salt bridges. The evolution of phosphorylation sites from glutamate and aspartate provides a rationale for why phosphorylation sometimes activates proteins, and helps explain the origins of this important and complex process.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Molecular evolution before the origin of species.Prog Biophys Mol Biol. 2002 May-Jul;79(1-3):77-133. doi: 10.1016/s0079-6107(02)00012-3. Prog Biophys Mol Biol. 2002. PMID: 12225777 Review.
-
Evolution and phyletic distribution of two-component signal transduction systems.Curr Opin Microbiol. 2010 Apr;13(2):219-25. doi: 10.1016/j.mib.2009.12.011. Epub 2010 Feb 3. Curr Opin Microbiol. 2010. PMID: 20133179 Free PMC article. Review.
-
Loops and repeats in proteins as footprints of molecular evolution.Biochemistry (Mosc). 2012 Dec;77(13):1487-99. doi: 10.1134/S000629791213007X. Biochemistry (Mosc). 2012. PMID: 23379524 Review.
-
Classification and Lineage Tracing of SH2 Domains Throughout Eukaryotes.Methods Mol Biol. 2017;1555:59-75. doi: 10.1007/978-1-4939-6762-9_4. Methods Mol Biol. 2017. PMID: 28092027
-
Complex salt bridges in proteins: statistical analysis of structure and function.J Mol Biol. 1995 Dec 8;254(4):761-70. doi: 10.1006/jmbi.1995.0653. J Mol Biol. 1995. PMID: 7500348
Cited by
-
Critical Functions of PP2A-Like Protein Phosphotases in Regulating Meiotic Progression.Front Cell Dev Biol. 2021 Feb 25;9:638559. doi: 10.3389/fcell.2021.638559. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33718377 Free PMC article. Review.
-
Phosphorylation of Human Choline Kinase Beta by Protein Kinase A: Its Impact on Activity and Inhibition.PLoS One. 2016 May 5;11(5):e0154702. doi: 10.1371/journal.pone.0154702. eCollection 2016. PLoS One. 2016. PMID: 27149373 Free PMC article.
-
FUS is phosphorylated by DNA-PK and accumulates in the cytoplasm after DNA damage.J Neurosci. 2014 Jun 4;34(23):7802-13. doi: 10.1523/JNEUROSCI.0172-14.2014. J Neurosci. 2014. PMID: 24899704 Free PMC article.
-
Pathogenic mutations of human phosphorylation sites affect protein-protein interactions.Nat Commun. 2024 Apr 11;15(1):3146. doi: 10.1038/s41467-024-46794-8. Nat Commun. 2024. PMID: 38605029 Free PMC article.
-
Effects of carboxyl-terminal methylation on holoenzyme function of the PP2A subfamily.Biochem Soc Trans. 2020 Oct 30;48(5):2015-2027. doi: 10.1042/BST20200177. Biochem Soc Trans. 2020. PMID: 33125487 Free PMC article. Review.
References
-
- Abascal F, Zardoya R, Posada D. ProtTest: selection of best-fit models of protein evolution. Bioinformatics. 2005;21:2104–2105. - PubMed
-
- Alonso A, Sasin J, Bottini N, Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J, Mustelin T. Protein tyrosine phosphatases in the human genome. Cell. 2004;117:699–711. - PubMed
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Ballif BA, Villen J, Beausoleil SA, Schwartz D, Gygi SP. Phosphoproteomic analysis of the developing mouse brain. Mol Cell Proteomics. 2004;3:1093–1101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

