Biogenesis of cbb(3)-type cytochrome c oxidase in Rhodobacter capsulatus
- PMID: 22079199
- PMCID: PMC3304015
- DOI: 10.1016/j.bbabio.2011.10.011
Biogenesis of cbb(3)-type cytochrome c oxidase in Rhodobacter capsulatus
Abstract
The cbb(3)-type cytochrome c oxidases (cbb(3)-Cox) constitute the second most abundant cytochrome c oxidase (Cox) group after the mitochondrial-like aa(3)-type Cox. They are present in bacteria only, and are considered to represent a primordial innovation in the domain of Eubacteria due to their phylogenetic distribution and their similarity to nitric oxide (NO) reductases. They are crucial for the onset of many anaerobic biological processes, such as anoxygenic photosynthesis or nitrogen fixation. In addition, they are prevalent in many pathogenic bacteria, and important for colonizing low oxygen tissues. Studies related to cbb(3)-Cox provide a fascinating paradigm for the biogenesis of sophisticated oligomeric membrane proteins. Complex subunit maturation and assembly machineries, producing the c-type cytochromes and the binuclear heme b(3)-Cu(B) center, have to be coordinated precisely both temporally and spatially to yield a functional cbb(3)-Cox enzyme. In this review we summarize our current knowledge on the structure, regulation and assembly of cbb(3)-Cox, and provide a highly tentative model for cbb(3)-Cox assembly and formation of its heme b(3)-Cu(B) binuclear center. This article is part of a Special Issue entitled: Biogenesis/Assembly of Respiratory Enzyme Complexes.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures

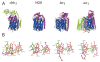
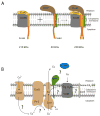

References
-
- Pereira MM, Santana M, Teixeira M. A novel scenario for the evolution of haem-copper oxygen reductases. Biochimica et biophysica acta. 2001;1505:185–208. - PubMed
-
- Flock U, Reimann J, Adelroth P. Proton transfer in bacterial nitric oxide reductase. Biochem Soc Trans. 2006;34:188–190. - PubMed
-
- Matoba S, Kang JG, Patino WD, Wragg A, Boehm M, Gavrilova O, Hurley PJ, Bunz F, Hwang PM. p53 regulates mitochondrial respiration. Science. 2006;312:1650–1653. - PubMed
-
- Won KY, Lim SJ, Kim GY, Kim YW, Han SA, Song JY, Lee DK. Regulatory role of p53 in cancer metabolism via SCO2 and TIGAR in human breast cancer. Hum Pathol. 2011 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

