Microarray analysis of CA1 pyramidal neurons in a mouse model of tauopathy reveals progressive synaptic dysfunction
- PMID: 22079237
- PMCID: PMC3259262
- DOI: 10.1016/j.nbd.2011.10.022
Microarray analysis of CA1 pyramidal neurons in a mouse model of tauopathy reveals progressive synaptic dysfunction
Abstract
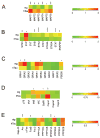
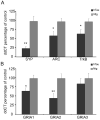
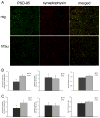
The hTau mouse model of tauopathy was utilized to assess gene expression changes in vulnerable hippocampal CA1 neurons. CA1 pyramidal neurons were microaspirated via laser capture microdissection followed by RNA amplification in combination with custom-designed microarray analysis and qPCR validation in hTau mice and nontransgenic (ntg) littermates aged 11-14months. Statistical analysis revealed ~8% of all the genes on the array platform were dysregulated, with notable downregulation of several synaptic-related markers including synaptophysin (Syp), synaptojanin, and synaptobrevin, among others. Downregulation was also observed for select glutamate receptors (GluRs), Psd-95, TrkB, and several protein phosphatase subunits. In contrast, upregulation of tau isoforms and a calpain subunit were found. Microarray assessment of synaptic-related markers in a separate cohort of hTau mice at 7-8months of age indicated only a few alterations compared to the 11-14month cohort, suggesting progressive synaptic dysfunction occurs as tau accumulates in CA1 pyramidal neurons. An assessment of SYP and PSD-95 expression was performed in the hippocampal CA1 sector of hTau and ntg mice via confocal laser scanning microscopy along with hippocampal immunoblot analysis for protein-based validation of selected microarray observations. Results indicate significant decreases in SYP-immunoreactive and PSD-95-immunoreactive puncta as well as downregulation of SYP-immunoreactive and PSD-95-immunoreactive band intensity in hTau mice compared to age-matched ntg littermates. In summary, the high prevalence of downregulation of synaptic-related genes indicates that the moderately aged hTau mouse may be a model of tau-induced synaptodegeneration, and has profound effects on how we perceive progressive tau pathology affecting synaptic transmission in AD.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

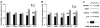
References
-
- ABI. Guide to Performing Relative Quantitation of Gene Expression Using Real-Time Quantitative PCR. Applied Biosystems Product Guide. 2004:1–60.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

