Protective immunity against mouse upper genital tract pathology correlates with high IFNγ but low IL-17 T cell and anti-secretion protein antibody responses induced by replicating chlamydial organisms in the airway
- PMID: 22079265
- PMCID: PMC3246108
- DOI: 10.1016/j.vaccine.2011.10.059
Protective immunity against mouse upper genital tract pathology correlates with high IFNγ but low IL-17 T cell and anti-secretion protein antibody responses induced by replicating chlamydial organisms in the airway
Abstract
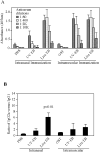
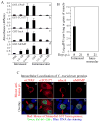
To search for optimal immunization conditions for inducing protective immunity against upper genital tract pathologies caused by chlamydial intravaginal infection, we compared protection efficacy in mice immunized intranasally or intramuscularly with live or inactivated Chlamydia muridarum organisms. Mice immunized intranasally with live organisms developed strong protection against both vaginal shedding of infectious organisms and upper genital tract pathologies. The protection correlated with a robust antigen-specific T cell response with high IFNγ but low IL-17. Although a significant level of IL-5 was also detected, these mice maintained an overall Th1-dorminant immunity following immunization and challenge infection. On the contrary, mice immunized intranasally with inactivated organisms or intramuscularly with live or inactivated organisms produced high levels of IL-17 and still developed significant upper genital tract pathologies. High titers of antibodies against chlamydial secretion antigens were detected only in mice immunized intranasally with live organisms but not mice in other groups, suggesting that the intranasally inoculated live organisms were able to undergo replication and immune responses to the chlamydial secretion proteins may contribute to protective immunity. These observations have provided important information on how to develop subunit vaccines for inducing protective immunity against urogenital infection with Chlamydia trachomatis organisms.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures




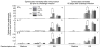
Similar articles
-
Intranasal vaccination with a secreted chlamydial protein enhances resolution of genital Chlamydia muridarum infection, protects against oviduct pathology, and is highly dependent upon endogenous gamma interferon production.Infect Immun. 2007 Feb;75(2):666-76. doi: 10.1128/IAI.01280-06. Epub 2006 Nov 21. Infect Immun. 2007. PMID: 17118987 Free PMC article.
-
Intranasal vaccination with Chlamydia pneumoniae induces cross-species immunity against genital Chlamydia muridarum challenge in mice.PLoS One. 2013 May 31;8(5):e64917. doi: 10.1371/journal.pone.0064917. Print 2013. PLoS One. 2013. PMID: 23741420 Free PMC article.
-
Immunization with chlamydial plasmid protein pORF5 DNA vaccine induces protective immunity against genital chlamydial infection in mice.Sci China C Life Sci. 2008 Nov;51(11):973-80. doi: 10.1007/s11427-008-0130-9. Epub 2008 Nov 7. Sci China C Life Sci. 2008. PMID: 18989639
-
Induction of protective immunity against Chlamydia muridarum intravaginal infection with the chlamydial immunodominant antigen macrophage infectivity potentiator.Microbes Infect. 2013 Apr;15(4):329-38. doi: 10.1016/j.micinf.2013.02.001. Epub 2013 Feb 14. Microbes Infect. 2013. PMID: 23416214 Free PMC article.
-
Vaccination against Chlamydia genital infection utilizing the murine C. muridarum model.Infect Immun. 2011 Mar;79(3):986-96. doi: 10.1128/IAI.00881-10. Epub 2010 Nov 15. Infect Immun. 2011. PMID: 21078844 Free PMC article. Review.
Cited by
-
Induction of Transmucosal Protection by Oral Vaccination with an Attenuated Chlamydia.Infect Immun. 2023 May 16;91(5):e0004323. doi: 10.1128/iai.00043-23. Epub 2023 Apr 10. Infect Immun. 2023. PMID: 37036335 Free PMC article.
-
Effects of Different Adjuvants on the Protective Efficacy of a Subcellular Vaccine Against Chlamydia abortus Infection in Sheep.Vaccines (Basel). 2025 Jun 5;13(6):609. doi: 10.3390/vaccines13060609. Vaccines (Basel). 2025. PMID: 40573940 Free PMC article.
-
Plasmid-Encoded Pgp5 Is a Significant Contributor to Chlamydia muridarum Induction of Hydrosalpinx.PLoS One. 2015 Apr 27;10(4):e0124840. doi: 10.1371/journal.pone.0124840. eCollection 2015. PLoS One. 2015. PMID: 25915629 Free PMC article.
-
Protective immunity against Chlamydia trachomatis can engage both CD4+ and CD8+ T cells and bridge the respiratory and genital mucosae.J Immunol. 2015 Mar 1;194(5):2319-29. doi: 10.4049/jimmunol.1402675. Epub 2015 Jan 30. J Immunol. 2015. PMID: 25637024 Free PMC article.
-
Reduced live organism recovery and lack of hydrosalpinx in mice infected with plasmid-free Chlamydia muridarum.Infect Immun. 2014 Mar;82(3):983-92. doi: 10.1128/IAI.01543-13. Epub 2013 Dec 16. Infect Immun. 2014. PMID: 24343644 Free PMC article.
References
-
- Centers for Disease Control and Prevention C; Services USDoHaH. Sexually Transmitted Disease Surveillance 2008. Atlanta, GA: Nov, 2009. http://www.cdc.gov/std/stats08/toc.htm.
-
- Rockey DD, Wang J, Lei L, Zhong G. Chlamydia vaccine candidates and tools for chlamydial antigen discovery. Expert Rev Vaccines. 2009 Oct;8(10):1365–77. - PubMed
-
- Sherman KJ, Daling JR, Stergachis A, Weiss NS, Foy HM, Wang SP, et al. Sexually transmitted diseases and tubal pregnancy. Sex Transm Dis. 1990 Jul-Sep;17(3):115–21. - PubMed
-
- Kinnunen AH, Surcel HM, Lehtinen M, Karhukorpi J, Tiitinen A, Halttunen M, et al. HLA DQ alleles and interleukin-10 polymorphism associated with Chlamydia trachomatis-related tubal factor infertility: a case-control study. Hum Reprod. 2002 Aug;17(8):2073–8. - PubMed
-
- Stephens RS. The cellular paradigm of chlamydial pathogenesis. Trends Microbiol. 2003 Jan;11(1):44–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

