Development of IgA nephropathy-like glomerulonephritis associated with Wiskott-Aldrich syndrome protein deficiency
- PMID: 22079330
- PMCID: PMC3273668
- DOI: 10.1016/j.clim.2011.10.001
Development of IgA nephropathy-like glomerulonephritis associated with Wiskott-Aldrich syndrome protein deficiency
Abstract
Wiskott-Aldrich syndrome (WAS) is a rare X-linked disorder caused by mutations in the WAS gene. Glomerulonephritis is a frequent complication, however, histopathological data from affected patients is scarce because the thrombocytopenia that affects most patients is a contraindication to renal biopsies. We found that WASp-deficient mice develop proliferative glomerulonephritis reminiscent of human IgA nephropathy (IgAN). We examined whether increased aberrant IgA production is associated with the development of glomerulonephritis in WASp-deficient mice. Serum IgA and IgA production by splenic B cells was increased in WASp-deficient mice compared to wild-type (WT) mice. A lectin-binding study revealed a reduced ratio of sialylated and galactosylated IgA in the sera from old WASp-deficient mice. Circulating IgA-containing immune complexes showed significantly higher titers in WASp-deficient mice compared to WT mice. These results indicate that the increased IgA production and aberrant glycosylation of IgA may be critically involved in the pathogenesis of glomerulonephritis in WAS.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no financial conflicts of interest to disclose.
Figures
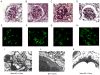
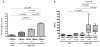


References
-
- Bosticardo M, Marangoni F, Aiuti A, Villa A, Grazia RM. Recent advances in understanding the pathophysiology of Wiskott-Aldrich syndrome. Blood. 2009;113:6288–6295. - PubMed
-
- Sullivan KE, Mullen CA, Blaese RM, Winkelstein JA. A multiinstitutional survey of the Wiskott-Aldrich syndrome. J Pediatr. 1994;125:876–885. - PubMed
-
- Dupuis-Girod S, Medioni J, Haddad E, Quartier P, Cavazzana-Calvo M, Le Deist F, de Saint Basile G, Delaunay J, Schwarz K, Casanova JL, Blanche S, Fischer A. Autoimmunity in Wiskott-Aldrich syndrome: risk factors, clinical features, and outcome in a single center cohort of 55 patients. Pediatrics. 2003;111:e622–627. - PubMed
-
- Schurman SH, Candotti F. Autoimmunity in Wiskott-Aldrich syndrome. Curr Opin Rheumatol. 2003;15:446–453. - PubMed
-
- Imai K, Morio T, Zhu Y, Jin Y, Itoh S, Kajiwara M, Yata J, Mizutani S, Ochs HD, Nonoyama S. Clinical course of patients with WASP gene mutations. Blood. 2004;103:456–464. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

