Phosphorylated smooth muscle heavy meromyosin shows an open conformation linked to activation
- PMID: 22079364
- PMCID: PMC3295741
- DOI: 10.1016/j.jmb.2011.10.047
Phosphorylated smooth muscle heavy meromyosin shows an open conformation linked to activation
Abstract
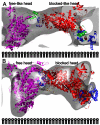
Smooth muscle myosin and smooth muscle heavy meromyosin (smHMM) are activated by regulatory light chain phosphorylation, but the mechanism remains unclear. Dephosphorylated, inactive smHMM assumes a closed conformation with asymmetric intramolecular head-head interactions between motor domains. The "free head" can bind to actin, but the actin binding interface of the "blocked head" is involved in interactions with the free head. We report here a three-dimensional structure for phosphorylated, active smHMM obtained using electron crystallography of two-dimensional arrays. Head-head interactions of phosphorylated smHMM resemble those found in the dephosphorylated state but occur between different molecules, not within the same molecule. The light chain binding domain structure of phosphorylated smHMM differs markedly from that of the "blocked" head of dephosphorylated smHMM. We hypothesize that regulatory light chain phosphorylation opens the inhibited conformation primarily by its effect on the blocked head. Singly phosphorylated smHMM is not compatible with the closed conformation if the blocked head is phosphorylated. This concept has implications for the extent of myosin activation at low levels of phosphorylation in smooth muscle.
Copyright © 2011. Published by Elsevier Ltd.
Figures





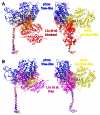
References
-
- Sellers JR, Sheterline P, editors. Protein Profile. 2 edit Oxford University Press; Oxford: 1999. Myosins.
-
- Geeves MA, Holmes KC. Structural mechanism of muscle contraction. Annu Rev Biochem. 1999;68:687–728. - PubMed
-
- Trybus KM. Regulation of smooth muscle myosin. Cell Motil Cytoskeleton. 1991;18:81–5. - PubMed
-
- Sellers JR. Mechanism of the phosphorylation-dependent regulation of smooth muscle heavy meromyosin. J Biol Chem. 1985;260:15815–9. - PubMed
-
- Ikebe M, Hartshorne DJ. The role of myosin phosphorylation in the contraction-relaxation cycle of smooth muscle. Experientia. 1985;41:1006–10. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

