Vitamin D regulation of immune function in the gut: why do T cells have vitamin D receptors?
- PMID: 22079836
- PMCID: PMC3781946
- DOI: 10.1016/j.mam.2011.10.014
Vitamin D regulation of immune function in the gut: why do T cells have vitamin D receptors?
Abstract
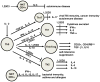
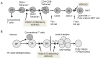
Low vitamin D status is associated with an increased risk of immune-mediated diseases like inflammatory bowel disease (IBD) in humans. Experimentally vitamin D status is a factor that shapes the immune response. Animals that are either vitamin D deficient or vitamin D receptor (VDR) deficient are prone to develop IBD. Conventional T cells develop normally in VDR knockout (KO) mice but over-produce IFN-γ and IL-17. Naturally occurring FoxP3+ regulatory T cells are present in normal numbers in VDR KO mice and function as well as wildtype T regs. Vitamin D and the VDR are required for the development and function of two regulatory populations of T cells that require non-classical MHC class 1 for development. The two vitamin D dependent cell types are the iNKT cells and CD4/CD8αα intraepithelial lymphocytes (IEL). Protective immune responses that depend on iNKT cells or CD8αα IEL are therefore impaired in the vitamin D or VDR deficient host and the mice are more susceptible to immune-mediated diseases in the gut.
Copyright © 2011. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare no financial or commercial conflict of interest.
Figures
References
-
- Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, Taniguchi M, Grusby MJ, DeKruyff RH, Umetsu DT. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9 (5):582–588. - PubMed
-
- Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, de Waal-Malefyt R, Coffman RL, Hawrylowicz CM, O’Garra A. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195 (5):603–616. - PMC - PubMed
-
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. - PubMed
-
- Benlagha K, Kyin T, Beavis A, Teyton L, Bendelac A. A thymic precursor to the NK T cell lineage. Science. 2002;296 (5567):553–555. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

