Neutrophils in innate host defense against Staphylococcus aureus infections
- PMID: 22080185
- PMCID: PMC3271231
- DOI: 10.1007/s00281-011-0295-3
Neutrophils in innate host defense against Staphylococcus aureus infections
Abstract
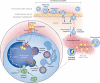
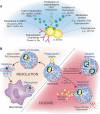
Staphylococcus aureus has been an important human pathogen throughout history and is currently a leading cause of bacterial infections worldwide. S. aureus has the unique ability to cause a continuum of diseases, ranging from minor skin infections to fatal necrotizing pneumonia. Moreover, the emergence of highly virulent, drug-resistant strains such as methicillin-resistant S. aureus in both healthcare and community settings is a major therapeutic concern. Neutrophils are the most prominent cellular component of the innate immune system and provide an essential primary defense against bacterial pathogens such as S. aureus. Neutrophils are rapidly recruited to sites of infection where they bind and ingest invading S. aureus, and this process triggers potent oxidative and non-oxidative antimicrobial killing mechanisms that serve to limit pathogen survival and dissemination. S. aureus has evolved numerous mechanisms to evade host defense strategies employed by neutrophils, including the ability to modulate normal neutrophil turnover, a process critical to the resolution of acute inflammation. Here we provide an overview of the role of neutrophils in host defense against bacterial pathogens and discuss strategies employed by S. aureus to circumvent neutrophil function.
Figures
References
-
- Diekema DJ, Pfaller MA, Schmitz FJ, Smayevsky J, Bell J, Jones RN, Beach M. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clin Infect Dis. 2001;32(Suppl 2):S114–S132. doi: 10.1086/320184. - DOI - PubMed
-
- Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298(15):1763–1771. doi: 10.1001/jama.298.15.1763. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

