Antimicrobial and cell-penetrating peptides induce lipid vesicle fusion by folding and aggregation
- PMID: 22080286
- PMCID: PMC3269571
- DOI: 10.1007/s00249-011-0771-7
Antimicrobial and cell-penetrating peptides induce lipid vesicle fusion by folding and aggregation
Abstract
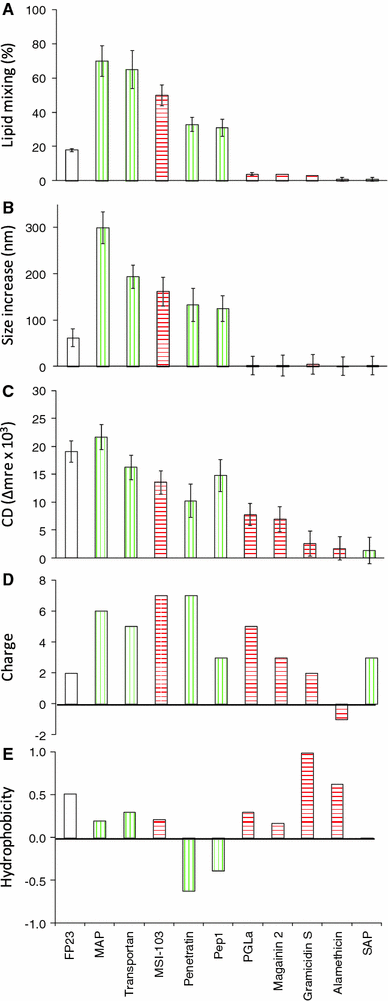
According to their distinct biological functions, membrane-active peptides are generally classified as antimicrobial (AMP), cell-penetrating (CPP), or fusion peptides (FP). The former two classes are known to have some structural and physicochemical similarities, but fusogenic peptides tend to have rather different features and sequences. Nevertheless, we found that many CPPs and some AMPs exhibit a pronounced fusogenic activity, as measured by a lipid mixing assay with vesicles composed of typical eukaryotic lipids. Compared to the HIV fusion peptide (FP23) as a representative standard, all designer-made peptides showed much higher lipid-mixing activities (MSI-103, MAP, transportan, penetratin, Pep1). Native sequences, on the other hand, were less fusogenic (magainin 2, PGLa, gramicidin S), and pre-aggregated ones were inactive (alamethicin, SAP). The peptide structures were characterized by circular dichroism before and after interacting with the lipid vesicles. A striking correlation between the extent of conformational change and the respective fusion activities was found for the series of peptides investigated here. At the same time, the CD data show that lipid mixing can be triggered by any type of conformation acquired upon binding, whether α-helical, β-stranded, or other. These observations suggest that lipid vesicle fusion can simply be driven by the energy released upon membrane binding, peptide folding, and possibly further aggregation. This comparative study of AMPs, CPPs, and FPs emphasizes the multifunctional aspects of membrane-active peptides, and it suggests that the origin of a peptide (native sequence or designer-made) may be more relevant to define its functional range than any given name.
Figures
Similar articles
-
Overlapping Properties of the Short Membrane-Active Peptide BP100 With (i) Polycationic TAT and (ii) α-helical Magainin Family Peptides.Front Cell Infect Microbiol. 2021 Apr 26;11:609542. doi: 10.3389/fcimb.2021.609542. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33981626 Free PMC article.
-
A critical evaluation of the conformational requirements of fusogenic peptides in membranes.Eur Biophys J. 2007 Apr;36(4-5):405-13. doi: 10.1007/s00249-006-0106-2. Epub 2006 Nov 7. Eur Biophys J. 2007. PMID: 17089152
-
Studies of membranotropic and fusogenic activity of two putative HCV fusion peptides.Biochim Biophys Acta Biomembr. 2019 Jan;1861(1):50-61. doi: 10.1016/j.bbamem.2018.10.011. Epub 2018 Oct 19. Biochim Biophys Acta Biomembr. 2019. PMID: 30343120
-
Cationic membrane peptides: atomic-level insight of structure-activity relationships from solid-state NMR.Amino Acids. 2013 Mar;44(3):821-33. doi: 10.1007/s00726-012-1421-9. Epub 2012 Oct 30. Amino Acids. 2013. PMID: 23108593 Free PMC article. Review.
-
Antimicrobial and Cell-Penetrating Peptides: How to Understand Two Distinct Functions Despite Similar Physicochemical Properties.Adv Exp Med Biol. 2019;1117:93-109. doi: 10.1007/978-981-13-3588-4_7. Adv Exp Med Biol. 2019. PMID: 30980355 Review.
Cited by
-
Solid-state nuclear magnetic resonance measurements of HIV fusion peptide 13CO to lipid 31P proximities support similar partially inserted membrane locations of the α helical and β sheet peptide structures.J Phys Chem A. 2013 Oct 3;117(39):9848-59. doi: 10.1021/jp312845w. Epub 2013 Feb 28. J Phys Chem A. 2013. PMID: 23418890 Free PMC article.
-
Peptide-membrane interactions of arginine-tryptophan peptides probed using quartz crystal microbalance with dissipation monitoring.Eur Biophys J. 2014 Jul;43(6-7):241-53. doi: 10.1007/s00249-014-0958-9. Epub 2014 Apr 18. Eur Biophys J. 2014. PMID: 24743917 Free PMC article.
-
Investigating Penetration and Antimicrobial Activity of Vector-Bicycle Conjugates.ACS Infect Dis. 2024 Jul 12;10(7):2381-2389. doi: 10.1021/acsinfecdis.3c00427. Epub 2024 Jun 12. ACS Infect Dis. 2024. PMID: 38865197 Free PMC article.
-
Novel non-helical antimicrobial peptides insert into and fuse lipid model membranes.Soft Matter. 2024 May 22;20(20):4088-4101. doi: 10.1039/d4sm00220b. Soft Matter. 2024. PMID: 38712559 Free PMC article.
-
Peptide-Membrane Interactions Monitored by Fluorescence Lifetime Imaging: A Study Case of Transportan 10.Langmuir. 2021 Nov 9;37(44):13148-13159. doi: 10.1021/acs.langmuir.1c02392. Epub 2021 Oct 29. Langmuir. 2021. PMID: 34714654 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

