The murine goblet cell protein mCLCA3 is a zinc-dependent metalloprotease with autoproteolytic activity
- PMID: 22080371
- PMCID: PMC3887686
- DOI: 10.1007/s10059-011-0158-8
The murine goblet cell protein mCLCA3 is a zinc-dependent metalloprotease with autoproteolytic activity
Abstract
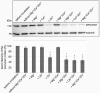
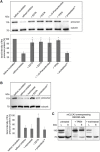
Several members of the CLCA family of proteins, originally named chloride channels, calcium-activated, have been shown to modulate chloride conductance in various cell types via an unknown mechanism. Moreover, the human (h) hCLCA1 is thought to modulate the severity of disease in asthma and cystic fibrosis (CF) patients. All CLCA proteins are post-translationally cleaved into two subunits, and recently, a conserved HEXXH zinc-binding amino acid motif has been identified, suggesting a role for CLCA proteins as metalloproteases. Here, we have characterized the cleavage and autoproteolytic activity of the murine model protein mCLCA3, which represents the murine orthologue of human hCLCA1. Using crude membrane fractions from transfected HEK293 cells, we demonstrate that mCLCA3 cleavage is zinc-dependent and exclusively inhibited by cation-chelating metalloprotease inhibitors. Cellular transport and secretion were not affected in response to a cleavage defect that was introduced by the insertion of an E157Q mutation within the HEXXH motif of mCLCA3. Interspecies conservation of these key results was further confirmed with the porcine (p) orthologue of hCLCA1 and mCLCA3, pCLCA1. Importantly, the mCLCA3E157Q mutant was cleaved after co-transfection with the wild-type mCLCA3 in HEK293 cells, suggesting that an intermolecular autoproteolytic event takes place. Edman degradation and MALDI-TOF-MS of the protein fragments identified a single cleavage site in mCLCA3 between amino acids 695 and 696. The data strongly suggest that secreted CLCA proteins have zinc-dependent autoproteolytic activity and that they may cleave additional proteins.
Figures




References
-
- Brouillard F., Bensalem N., Hinzpeter A., Tondelier D., Trudel S., Gruber A.D., Ollero M., Edelman A. Blue native- SDS PAGE analysis reveals reduced expression of the mClCA3 protein in cystic fibrosis knock-out mice. Mol. Cell. Proteomics. (2005);4:1762–1775. - PubMed
-
- Cao J., Rehemtulla A., Pavlaki M., Kozarekar P., Chiarelli C. Furin directly cleaves proMMP-2 in the trans-Golgi network resulting in a nonfunctioning proteinase. J. Biol. Chem. (2005);280:10974–10980. - PubMed
-
- Contin C., Pitard V., Itai T., Nagata S., Moreau J.F., Dechanet-Merville J. Membrane-anchored CD40 is processed by the tumor necrosis factor-alpha-converting enzyme. Implications for CD40 signaling. J. Biol. Chem. (2003);278:32801–32809. - PubMed
-
- Creemers J.W., Vey M., Schafer W., Ayoubi T.A., Roebroek A.J., Klenk H.D., Garten W., Van de Ven W.J. Endoproteolytic cleavage of its propeptide is a prerequisite for efficient transport of furin out of the endoplasmic reticulum. J. Biol. Chem. (1995);270:2695–2702. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

