T cell protein tyrosine phosphatase attenuates T cell signaling to maintain tolerance in mice
- PMID: 22080863
- PMCID: PMC3226006
- DOI: 10.1172/JCI59492
T cell protein tyrosine phosphatase attenuates T cell signaling to maintain tolerance in mice
Erratum in
-
T cell protein tyrosine phosphatase attenuates T cell signaling to maintain tolerance in mice.J Clin Invest. 2023 Oct 16;133(20):e175163. doi: 10.1172/JCI175163. J Clin Invest. 2023. PMID: 37843283 Free PMC article. No abstract available.
Abstract
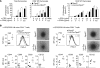
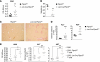
Many autoimmune diseases exhibit familial aggregation, indicating that they have genetic determinants. Single nucleotide polymorphisms in PTPN2, which encodes T cell protein tyrosine phosphatase (TCPTP), have been linked with the development of several autoimmune diseases, including type 1 diabetes and Crohn's disease. In this study, we have identified TCPTP as a key negative regulator of TCR signaling, which might explain the association of PTPN2 SNPs with autoimmune disease. We found that TCPTP dephosphorylates and inactivates Src family kinases to regulate T cell responses. Using T cell-specific TCPTP-deficient mice, we established that TCPTP attenuates T cell activation and proliferation in vitro and blunts antigen-induced responses in vivo. TCPTP deficiency lowered the in vivo threshold for TCR-dependent CD8(+) T cell proliferation. Consistent with this, T cell-specific TCPTP-deficient mice developed widespread inflammation and autoimmunity that was transferable to wild-type recipient mice by CD8(+) T cells alone. This autoimmunity was associated with increased serum levels of proinflammatory cytokines and anti-nuclear antibodies, T cell infiltrates in non-lymphoid tissues, and liver disease. These data indicate that TCPTP is a critical negative regulator of TCR signaling that sets the threshold for TCR-induced naive T cell responses to prevent autoimmune and inflammatory disorders arising.
Figures









Comment in
-
Unraveling the functional implications of GWAS: how T cell protein tyrosine phosphatase drives autoimmune disease.J Clin Invest. 2011 Dec;121(12):4618-21. doi: 10.1172/JCI60001. Epub 2011 Nov 14. J Clin Invest. 2011. PMID: 22080861 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

