The myriad roles of cyclic AMP in microbial pathogens: from signal to sword
- PMID: 22080930
- PMCID: PMC3785115
- DOI: 10.1038/nrmicro2688
The myriad roles of cyclic AMP in microbial pathogens: from signal to sword
Abstract
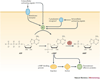
All organisms must sense and respond to their external environments, and this signal transduction often involves second messengers such as cyclic nucleotides. One such nucleotide is cyclic AMP, a universal second messenger that is used by diverse forms of life, including mammals, fungi, protozoa and bacteria. In this review, we discuss the many roles of cAMP in bacterial, fungal and protozoan pathogens and its contributions to microbial pathogenesis. These roles include the coordination of intracellular processes, such as virulence gene expression, with extracellular signals from the environment, and the manipulation of host immunity by increasing cAMP levels in host cells during infection.
Figures





References
-
- Beavo JA, Brunton LL. Cyclic nucleotide research -- still expanding after half a century. Nat Rev Mol Cell Biol. 2002;3:710–718. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

