Preemptive dosing of plerixafor given to poor stem cell mobilizers on day 5 of G-CSF administration
- PMID: 22080963
- PMCID: PMC3877677
- DOI: 10.1038/bmt.2011.217
Preemptive dosing of plerixafor given to poor stem cell mobilizers on day 5 of G-CSF administration
Abstract
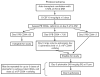
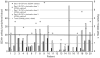
Plerixafor, given on day 4 of G-CSF treatment is more effective than G-CSF alone in mobilizing hematopoietic progenitor cells. We tested a strategy of preemptive plerixafor use following assessment of the peak mobilization response to 5 days of G-CSF. Patients were eligible for plerixafor if, on day 5 of G-CSF, there were <7 circulating CD34+ cells/μL or if <1.3 × 10(6) CD34+ cells/kg were collected on the first day of apheresis. Plerixafor (0.24 mg/kg s.c.) was given on day 5 of G-CSF followed by apheresis on day 6. This was repeated for up to two additional doses of plerixafor. The primary end point of the study was the percentage of patients who collected at least 2 × 10(6) CD34+ cells/kg. Twenty candidates for auto-SCT enrolled on the trial. The circulating CD34+ cell level increased a median of 3.1 fold (range 1-8 fold) after the first dose of plerixafor and a median of 1.2 fold (range 0.3-6.5 fold) after the second dose of plerixafor. In all, 15 out of 20 (75%) patients achieved the primary end point. In conclusion, the decision to administer plerixafor can be delayed until after the peak mobilization response to G-CSF has been fully assessed.
Conflict of interest statement
N.C. and M.H. have relevant financial relationships as follows; Genzyme, Research Funding (N.C), and Genzyme, Honoraria and Research Funding (M.H.). Other authors have no relevant financial relationship to disclose.
Figures
Similar articles
-
Pharmacokinetics and pharmacodynamics of plerixafor in patients with non-Hodgkin lymphoma and multiple myeloma.Biol Blood Marrow Transplant. 2009 Jan;15(1):39-46. doi: 10.1016/j.bbmt.2008.10.018. Biol Blood Marrow Transplant. 2009. PMID: 19135941 Clinical Trial.
-
Plerixafor plus granulocyte colony-stimulating factor versus placebo plus granulocyte colony-stimulating factor for mobilization of CD34(+) hematopoietic stem cells in patients with multiple myeloma and low peripheral blood CD34(+) cell count: results of a subset analysis of a randomized trial.Biol Blood Marrow Transplant. 2012 Oct;18(10):1564-72. doi: 10.1016/j.bbmt.2012.05.017. Epub 2012 Jun 6. Biol Blood Marrow Transplant. 2012. PMID: 22683613 Clinical Trial.
-
Patients' outcome after rescue plerixafor administration for autologous stem cell mobilization: a single-center retrospective analysis.Transfusion. 2017 Jan;57(1):115-121. doi: 10.1111/trf.13883. Epub 2016 Nov 18. Transfusion. 2017. PMID: 27859332
-
Plerixafor: A chemokine receptor-4 antagonist for mobilization of hematopoietic stem cells for transplantation after high-dose chemotherapy for non-Hodgkin's lymphoma or multiple myeloma.Clin Ther. 2010 May;32(5):821-43. doi: 10.1016/j.clinthera.2010.05.007. Clin Ther. 2010. PMID: 20685493 Review.
-
Poor mobilizer and its countermeasures.Transfus Apher Sci. 2018 Oct;57(5):623-627. doi: 10.1016/j.transci.2018.09.007. Epub 2018 Sep 11. Transfus Apher Sci. 2018. PMID: 30268541 Review.
Cited by
-
Clinical experience with plerixafor as a mobilization regimen for autologous peripheral blood stem cell transplantation in patients with refractory germ cell tumors.Mol Clin Oncol. 2014 Nov;2(6):923-926. doi: 10.3892/mco.2014.362. Epub 2014 Jul 29. Mol Clin Oncol. 2014. PMID: 25279175 Free PMC article.
-
Phase 2 trial of intravenously administered plerixafor for stem cell mobilization in patients with multiple myeloma following lenalidomide-based initial therapy.Bone Marrow Transplant. 2014 Feb;49(2):201-5. doi: 10.1038/bmt.2013.175. Epub 2013 Nov 4. Bone Marrow Transplant. 2014. PMID: 24185588 Free PMC article. Clinical Trial.
-
Plerixafor in patients with lymphoma and multiple myeloma: effectiveness in cases with very low circulating CD34+ cell levels and preemptive intervention vs remobilization.Bone Marrow Transplant. 2015 Jan;50(1):34-9. doi: 10.1038/bmt.2014.196. Epub 2014 Sep 15. Bone Marrow Transplant. 2015. PMID: 25222503 Clinical Trial.
-
G-CSF + plerixafor versus G-CSF alone mobilized hematopoietic stem cells in patients with multiple myeloma and lymphoma: a systematic review and meta-analysis.Ann Med. 2024 Dec;56(1):2329140. doi: 10.1080/07853890.2024.2329140. Epub 2024 Mar 12. Ann Med. 2024. PMID: 38470973 Free PMC article.
-
Early measurement of CD34+ cells in peripheral blood after cyclophosphamide and granulocyte colony-stimulating factor treatment predicts later CD34+ mobilisation failure and is a possible criterion for guiding "on demand" use of plerixafor.Blood Transfus. 2013 Jan;11(1):94-101. doi: 10.2450/2012.0004-12. Epub 2012 Oct 10. Blood Transfus. 2013. PMID: 23114516 Free PMC article.
References
-
- DiPersio JF, Micallef IN, Stiff PJ, Bolwell BJ, Maziarz RT, Jacobsen E, et al. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin’s lymphoma. J Clin Oncol. 2009;27(28):4767–73. - PubMed
-
- DiPersio JF, Stadtmauer EA, Nademanee A, Micallef IN, Stiff PJ, Kaufman JL, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113(23):5720–6. - PubMed
-
- Gordan LN, Sugrue MW, Lynch JW, Williams KD, Khan SA, Wingard JR, et al. Poor mobilization of peripheral blood stem cells is a risk factor for worse outcome in lymphoma patients undergoing autologous stem cell transplantation. Leuk Lymphoma. 2003;44(5):815–20. - PubMed
-
- Kuittinen T, Nousiainen T, Halonen P, Mahlamaki E, Jantunen E. Prediction of mobilisation failure in patients with non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2004;33(9):907–12. - PubMed
-
- Akhtar S, Weshi AE, Rahal M, Khafaga Y, Tbakhi A, Humaidan H, et al. Factors affecting autologous peripheral blood stem cell collection in patients with relapsed or refractory diffuse large cell lymphoma and Hodgkin lymphoma: a single institution result of 168 patients. Leuk Lymphoma. 2008;49(4):769–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical