Profilin1 is required for glial cell adhesion and radial migration of cerebellar granule neurons
- PMID: 22081137
- PMCID: PMC3246249
- DOI: 10.1038/embor.2011.211
Profilin1 is required for glial cell adhesion and radial migration of cerebellar granule neurons
Abstract
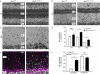
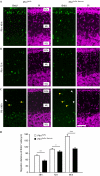
Cerebellar granule neurons (CGNs) exploit Bergmann glia (BG) fibres for radial migration, and cell-cell contacts have a pivotal role in this process. Nevertheless, little is known about the mechanisms that control CGN-BG interaction. Here we demonstrate that the actin-binding protein profilin1 is essential for CGN-glial cell adhesion and radial migration. Profilin1 ablation from mouse brains leads to a cerebellar hypoplasia, aberrant organization of cerebellar cortex layers and ectopic CGNs. Conversely, neuronal progenitor proliferation, tangential migration of neurons and BG morphology appear to be independent of profilin1. Our mouse data and the mapping of developmental neuropathies to the chromosomal region of PFN1 suggest a similar function for profilin1 in humans.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Adams NC, Tomoda T, Cooper M, Dietz G, Hatten ME (2002) Mice that lack astrotactin have slowed neuronal migration. Development 129: 965–972 - PubMed
-
- Ayala R, Shu T, Tsai LH (2007) Trekking across the brain: the journey of neuronal migration. Cell 128: 29–43 - PubMed
-
- Bershadsky A (2004) Magic touch: how does cell–cell adhesion trigger actin assembly? Trends Cell Biol 14: 589–593 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

