Superinfection in malaria: Plasmodium shows its iron will
- PMID: 22081142
- PMCID: PMC3245699
- DOI: 10.1038/embor.2011.213
Superinfection in malaria: Plasmodium shows its iron will
Abstract
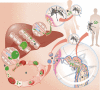
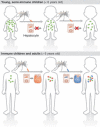
After the bite of a malaria-infected mosquito, the Plasmodium sporozoite infects liver cells and produces thousands of merozoites, which then infect red blood cells, causing malaria. In malaria-endemic areas, several hundred infected mosquitoes can bite an individual each year, increasing the risk of superinfection. However, in infants that are yet to acquire immunity, superinfections are infrequent. We have recently shown that blood-stage parasitaemia, above a minimum threshold, impairs the growth of a subsequent sporozoite infection of liver cells. Blood-stage parasites stimulate the production of the host iron-regulatory factor hepcidin, which redistributes iron away from hepatocytes, reducing the development of the iron-dependent liver stage. This could explain why Plasmodium superinfection is not often found in young nonimmune children. Here, we discuss the impact that such protection from superinfection might have in epidemiological settings or in programmes for controlling malaria, as well as how the induction of hepcidin and redistribution of iron might influence anaemia and the outcome of non-Plasmodium co-infections.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





Similar articles
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Host-mediated regulation of superinfection in malaria.Nat Med. 2011 Jun;17(6):732-7. doi: 10.1038/nm.2368. Epub 2011 May 15. Nat Med. 2011. PMID: 21572427 Free PMC article.
-
Cross-species regulation of malaria parasitaemia in the human host.Curr Opin Microbiol. 2002 Aug;5(4):431-7. doi: 10.1016/s1369-5274(02)00348-x. Curr Opin Microbiol. 2002. PMID: 12160865 Review.
-
Malaria parasite development in mosquitoes.Annu Rev Entomol. 1998;43:519-43. doi: 10.1146/annurev.ento.43.1.519. Annu Rev Entomol. 1998. PMID: 9444756 Review.
-
Parasite Recognition and Signaling Mechanisms in Innate Immune Responses to Malaria.Front Immunol. 2018 Dec 19;9:3006. doi: 10.3389/fimmu.2018.03006. eCollection 2018. Front Immunol. 2018. PMID: 30619355 Free PMC article. Review.
Cited by
-
Dynamical malaria models reveal how immunity buffers effect of climate variability.Proc Natl Acad Sci U S A. 2015 Jul 14;112(28):8786-91. doi: 10.1073/pnas.1419047112. Epub 2015 Jun 29. Proc Natl Acad Sci U S A. 2015. PMID: 26124134 Free PMC article.
-
Modulation of Iron Metabolism in Response to Infection: Twists for All Tastes.Pharmaceuticals (Basel). 2018 Sep 1;11(3):84. doi: 10.3390/ph11030084. Pharmaceuticals (Basel). 2018. PMID: 30200471 Free PMC article. Review.
-
Malaria blood stage infection suppresses liver stage infection via host-induced interferons but not hepcidin.Nat Commun. 2024 Mar 7;15(1):2104. doi: 10.1038/s41467-024-46270-3. Nat Commun. 2024. PMID: 38453916 Free PMC article.
-
From multiplicity of infection to force of infection for sparsely sampled Plasmodium falciparum populations at high transmission.medRxiv [Preprint]. 2025 Mar 19:2024.02.12.24302148. doi: 10.1101/2024.02.12.24302148. medRxiv. 2025. PMID: 38853963 Free PMC article. Preprint.
-
Cross-Talk Between Iron and Glucose Metabolism in the Establishment of Disease Tolerance.Front Immunol. 2018 Oct 30;9:2498. doi: 10.3389/fimmu.2018.02498. eCollection 2018. Front Immunol. 2018. PMID: 30425714 Free PMC article. Review.
References
-
- Abboud S, Haile DJ (2000) A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem 275: 19906–19912 - PubMed
-
- al-Yaman F, Genton B, Reeder JC, Anders RF, Smith T, Alpers MP (1997) Reduced risk of clinical malaria in children infected with multiple clones of Plasmodium falciparum in a highly endemic area: a prospective community study. Trans R Soc Trop Med Hyg 91: 602–605 - PubMed
-
- Armitage AE, Eddowes LA, Gileadi U, Cole S, Spottiswoode N, Selvakumar TA, Ho LP, Townsend AR, Drakesmith H (2011) Hepcidin regulation by innate immune and infectious stimuli. Blood 118: 4129–4139 - PubMed
-
- Babiker HA, Creasey AM, Bayoumi RA, Walliker D, Arnot DE (1991) Genetic diversity of Plasmodium falciparum in a village in eastern Sudan. 2. Drug resistance, molecular karyotypes and the mdr1 genotype of recent isolates. Trans R Soc Trop Med Hyg 85: 578–583 - PubMed
-
- Beier MS, Schwartz IK, Beier JC, Perkins PV, Onyango F, Koros JK, Campbell GH, Andrysiak PM, Brandling-Bennett AD (1988) Identification of malaria species by ELISA in sporozoite and oocyst infected Anopheles from western Kenya. Am J Trop Med Hyg 39: 323–327 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

