CD8αα and -αβ isotypes are equally recruited to the immunological synapse through their ability to bind to MHC class I
- PMID: 22081144
- PMCID: PMC3245696
- DOI: 10.1038/embor.2011.209
CD8αα and -αβ isotypes are equally recruited to the immunological synapse through their ability to bind to MHC class I
Abstract
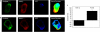
Bimolecular fluorescence complementation was used to engineer CD8 molecules so that CD8αα and CD8αβ dimers can be independently visualized on the surface of a T cell during antigen recognition. Using this approach, we show that CD8αα is recruited to the immunological synapse almost as well as CD8αβ, but because the kinase Lck associates preferentially with CD8αβ in lipid rafts, CD8αα is the weaker co-receptor. During recognition of the strong CD8αα ligand H2-TL, CD8αα is preferentially recruited. Thus, recruitment of the two CD8 species correlates with their relative binding to the available ligands, rather than with the co-receptor functions of the CD8 species.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


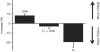
Similar articles
-
The murine CD94/NKG2 ligand, Qa-1b, is a high-affinity, functional ligand for the CD8αα homodimer.J Biol Chem. 2020 Mar 6;295(10):3239-3246. doi: 10.1074/jbc.RA119.010509. Epub 2020 Jan 28. J Biol Chem. 2020. PMID: 31992596 Free PMC article.
-
The CD8 isoform CD8alphaalpha is not a functional homologue of the TCR co-receptor CD8alphabeta.Curr Opin Immunol. 2004 Jun;16(3):264-70. doi: 10.1016/j.coi.2004.03.015. Curr Opin Immunol. 2004. PMID: 15134773 Review.
-
Late postnatal expansion of self-reactive CD8alphaalpha+ intestinal intraepithelial lymphocytes in mice.Autoimmunity. 2004 Dec;37(8):537-47. doi: 10.1080/08916930400027094. Autoimmunity. 2004. PMID: 15763916
-
MHC class I allele dosage alters CD8 expression by intestinal intraepithelial lymphocytes.J Immunol. 2001 Sep 1;167(5):2561-8. doi: 10.4049/jimmunol.167.5.2561. J Immunol. 2001. PMID: 11509596
-
Doubting the TCR coreceptor function of CD8alphaalpha.Immunity. 2008 Feb;28(2):149-59. doi: 10.1016/j.immuni.2008.01.005. Immunity. 2008. PMID: 18275828 Review.
Cited by
-
CD8αα+T cells exert a pro-inflammatory role in patients with psoriasis.Skin Health Dis. 2021 Nov 16;1(4):e64. doi: 10.1002/ski2.64. eCollection 2021 Dec. Skin Health Dis. 2021. PMID: 35663772 Free PMC article.
-
A ternary complex comprising transportin1, Rab8 and the ciliary targeting signal directs proteins to ciliary membranes.J Cell Sci. 2016 Oct 15;129(20):3922-3934. doi: 10.1242/jcs.194019. Epub 2016 Sep 15. J Cell Sci. 2016. PMID: 27633000 Free PMC article.
-
The kinase occupancy of T cell coreceptors reconsidered.Proc Natl Acad Sci U S A. 2022 Dec 6;119(49):e2213538119. doi: 10.1073/pnas.2213538119. Epub 2022 Dec 1. Proc Natl Acad Sci U S A. 2022. PMID: 36454761 Free PMC article.
-
The molecular determinants of CD8 co-receptor function.Immunology. 2012 Oct;137(2):139-48. doi: 10.1111/j.1365-2567.2012.03625.x. Immunology. 2012. PMID: 22804746 Free PMC article. Review.
-
Initiation of TCR phosphorylation and signal transduction.Front Immunol. 2011 Dec 7;2:72. doi: 10.3389/fimmu.2011.00072. eCollection 2011. Front Immunol. 2011. PMID: 22566861 Free PMC article.
References
-
- Arcaro A, Gregoire C, Boucheron N, Stotz S, Palmer E, Malissen B, Luescher IF (2000) Essential role of CD8 palmitoylation in CD8 coreceptor function. J Immunol 165: 2068–2076 - PubMed
-
- Attinger A, Devine L, Wang-Zhu Y, Martin D, Wang JH, Reinherz EL, Kronenberg M, Cheroutre H, Kavathas P (2005) Molecular basis for the high affinity interaction between the thymic leukemia antigen and the CD8αα molecule. J Immunol 174: 3501–3507 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

