Cj1386 is an ankyrin-containing protein involved in heme trafficking to catalase in Campylobacter jejuni
- PMID: 22081390
- PMCID: PMC3256678
- DOI: 10.1128/JB.05740-11
Cj1386 is an ankyrin-containing protein involved in heme trafficking to catalase in Campylobacter jejuni
Abstract
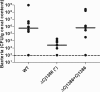
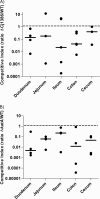
Campylobacter jejuni, a microaerophilic bacterium, is the most frequent cause of human bacterial gastroenteritis. C. jejuni is exposed to harmful reactive oxygen species (ROS) produced during its own normal metabolic processes and during infection from the host immune system and from host intestinal microbiota. These ROS will damage DNA and proteins and cause peroxidation of lipids. Consequently, identifying ROS defense mechanisms is important for understanding how Campylobacter survives this environmental stress during infection. Construction of a ΔCj1386 isogenic deletion mutant and phenotypic assays led to its discovery as a novel oxidative stress defense gene. The ΔCj1386 mutant has an increased sensitivity toward hydrogen peroxide. The Cj1386 gene is located directly downstream from katA (catalase) in the C. jejuni genome. A ΔkatAΔ Cj1386 double deletion mutant was constructed and exhibited a sensitivity to hydrogen peroxide similar to that seen in the ΔCj1386 and ΔkatA single deletion mutants. This observation suggests that Cj1386 may be involved in the same detoxification pathway as catalase. Despite identical KatA abundances, catalase activity assays showed that the ΔCj1386 mutant had a reduced catalase activity relative to that of wild-type C. jejuni. Heme quantification of KatA protein from the ΔCj1386 mutant revealed a significant decrease in heme concentration. This indicates an important role for Cj1386 in heme trafficking to KatA within C. jejuni. Interestingly, the ΔCj1386 mutant had a reduced ability to colonize the ceca of chicks and was outcompeted by the wild-type strain for colonization of the gastrointestinal tract of neonate piglets. These results indicate an important role for Cj1386 in Campylobacter colonization and pathogenesis.
Figures





Similar articles
-
Cj1386, an atypical hemin-binding protein, mediates hemin trafficking to KatA in Campylobacter jejuni.J Bacteriol. 2015 Mar;197(5):1002-11. doi: 10.1128/JB.02346-14. Epub 2014 Dec 29. J Bacteriol. 2015. PMID: 25548249 Free PMC article.
-
The PAS Domain-Containing Protein HeuR Regulates Heme Uptake in Campylobacter jejuni.mBio. 2016 Nov 15;7(6):e01691-16. doi: 10.1128/mBio.01691-16. mBio. 2016. PMID: 27935836 Free PMC article.
-
High-throughput sequencing of Campylobacter jejuni insertion mutant libraries reveals mapA as a fitness factor for chicken colonization.J Bacteriol. 2014 Jun;196(11):1958-67. doi: 10.1128/JB.01395-13. Epub 2014 Mar 14. J Bacteriol. 2014. PMID: 24633877 Free PMC article.
-
Re-thinking the chicken-Campylobacter jejuni interaction: a review.Avian Pathol. 2018 Aug;47(4):352-363. doi: 10.1080/03079457.2018.1475724. Epub 2018 Jun 11. Avian Pathol. 2018. PMID: 29764197 Review.
-
A tolerogenic mucosal immune response leads to persistent Campylobacter jejuni colonization in the chicken gut.Crit Rev Microbiol. 2012 Feb;38(1):17-29. doi: 10.3109/1040841X.2011.615298. Epub 2011 Oct 13. Crit Rev Microbiol. 2012. PMID: 21995731 Review.
Cited by
-
Posttranscriptional Regulation in Response to Different Environmental Stresses in Campylobacter jejuni.Microbiol Spectr. 2022 Jun 29;10(3):e0020322. doi: 10.1128/spectrum.00203-22. Epub 2022 Jun 9. Microbiol Spectr. 2022. PMID: 35678555 Free PMC article.
-
Low and high concentrations of butyrate regulate fat accumulation in chicken adipocytes via different mechanisms.Adipocyte. 2020 Dec;9(1):120-131. doi: 10.1080/21623945.2020.1738791. Adipocyte. 2020. PMID: 32163011 Free PMC article.
-
Heme homeostasis and its regulation by hemoproteins in bacteria.mLife. 2024 Jul 11;3(3):327-342. doi: 10.1002/mlf2.12120. eCollection 2024 Sep. mLife. 2024. PMID: 39359680 Free PMC article. Review.
-
Genes important for catalase activity in Enterococcus faecalis.PLoS One. 2012;7(5):e36725. doi: 10.1371/journal.pone.0036725. Epub 2012 May 10. PLoS One. 2012. PMID: 22590595 Free PMC article.
-
Campylobacter jejuni transcriptome changes during loss of culturability in water.PLoS One. 2017 Nov 30;12(11):e0188936. doi: 10.1371/journal.pone.0188936. eCollection 2017. PLoS One. 2017. PMID: 29190673 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

