Functions of the duplicated hik31 operons in central metabolism and responses to light, dark, and carbon sources in Synechocystis sp. strain PCC 6803
- PMID: 22081400
- PMCID: PMC3256662
- DOI: 10.1128/JB.06207-11
Functions of the duplicated hik31 operons in central metabolism and responses to light, dark, and carbon sources in Synechocystis sp. strain PCC 6803
Abstract
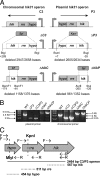
There are two closely related hik31 operons involved in signal transduction on the chromosome and the pSYSX plasmid in the cyanobacterium Synechocystis sp. strain PCC 6803. We studied the growth, cell morphology, and gene expression in operon and hik mutants for both copies, under different growth conditions, to examine whether the duplicated copies have the same or different functions and gene targets and whether they are similarly regulated. Phenotype analysis suggested that both operons regulated common and separate targets in the light and the dark. The chromosomal operon was involved in the negative control of autotrophic events, whereas the plasmid operon was involved in the positive control of heterotrophic events. Both the plasmid and double operon mutant cells were larger and had division defects. The growth data also showed a regulatory role for the chromosomal hik gene under high-CO(2) conditions and the plasmid operon under low-O(2) conditions. Metal stress experiments indicated a role for the chromosomal hik gene and operon in mediating Zn and Cd tolerance, the plasmid operon in Co tolerance, and the chromosomal operon and plasmid hik gene in Ni tolerance. We conclude that both operons are differentially and temporally regulated. We suggest that the chromosomal operon is the primarily expressed copy and the plasmid operon acts as a backup to maintain appropriate gene dosages. Both operons share an integrated regulatory relationship and are induced in high light, in glucose, and in active cell growth. Additionally, the plasmid operon is induced in the dark with or without glucose.
Figures







References
-
- Alves R, Savageau MA. 2003. Comparative analysis of prototype two-component systems with either bifunctional or monofunctional sensors: differences in molecular structure and physiological function. Mol. Microbiol. 48:25–51 - PubMed
-
- Buelow DR, Raivio TL. 2010. Three (and more) component regulatory systems—auxiliary regulators of bacterial histidine kinases. Mol. Microbiol. 75:547–566 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

