Fat-derived factor omentin stimulates endothelial cell function and ischemia-induced revascularization via endothelial nitric oxide synthase-dependent mechanism
- PMID: 22081609
- PMCID: PMC3249092
- DOI: 10.1074/jbc.M111.261818
Fat-derived factor omentin stimulates endothelial cell function and ischemia-induced revascularization via endothelial nitric oxide synthase-dependent mechanism
Abstract
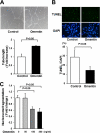
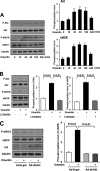
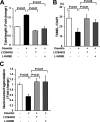
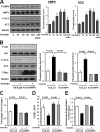
Obesity-related diseases are associated with vascular dysfunction and impaired revascularization. Omentin is a fat-derived secreted protein, which is down-regulated in association with obese complications. Here, we investigated whether omentin modulates endothelial cell function and revascularization processes in vitro and in vivo. Systemic delivery of an adenoviral vector expressing omentin (Ad-omentin) enhanced blood flow recovery and capillary density in ischemic limbs of wild-type mice in vivo, which were accompanied by increased phosphorylation of Akt and endothelial nitric oxide synthase (eNOS). In cultured human umbilical vein endothelial cells (HUVECs), a physiological concentration of recombinant omentin protein increased differentiation into vascular-like structures and decreased apoptotic activity under conditions of serum starvation. Treatment with omentin protein stimulated the phosphorylation of Akt and eNOS in HUVECs. Inhibition of Akt signaling by treatment with dominant-negative Akt or LY294002 blocked the stimulatory effects of omentin on differentiation and survival of HUVECs and reversed omentin-stimulated eNOS phosphorylation. Pretreatment with the NOS inhibitor also reduced the omentin-induced increase in HUVEC differentiation and survival. Omentin protein also stimulated the phosphorylation of AMP-activated protein kinase in HUVECs. Transduction with dominant-negative AMP-activated protein kinase diminished omentin-induced phosphorylation of Akt and omentin-stimulated increase in HUVEC differentiation and survival. Of importance, in contrast to wild-type mice, systemic administration of Ad-omentin did not affect blood flow in ischemic muscle in eNOS-deficient mice in vivo. These data indicate that omentin promotes endothelial cell function and revascularization in response to ischemia through its ability to stimulate an Akt-eNOS signaling pathway.
Figures


Similar articles
-
Vildagliptin stimulates endothelial cell network formation and ischemia-induced revascularization via an endothelial nitric-oxide synthase-dependent mechanism.J Biol Chem. 2014 Sep 26;289(39):27235-27245. doi: 10.1074/jbc.M114.557835. Epub 2014 Aug 6. J Biol Chem. 2014. PMID: 25100725 Free PMC article.
-
Diallyl Trisulfide Augments Ischemia-Induced Angiogenesis via an Endothelial Nitric Oxide Synthase-Dependent Mechanism.Circ J. 2017 May 25;81(6):870-878. doi: 10.1253/circj.CJ-16-1097. Epub 2017 Feb 17. Circ J. 2017. PMID: 28216514
-
Omentin protects against LPS-induced ARDS through suppressing pulmonary inflammation and promoting endothelial barrier via an Akt/eNOS-dependent mechanism.Cell Death Dis. 2016 Sep 8;7(9):e2360. doi: 10.1038/cddis.2016.265. Cell Death Dis. 2016. PMID: 27607575 Free PMC article.
-
Omentin-1: Protective impact on ischemic stroke via ameliorating atherosclerosis.Clin Chim Acta. 2021 Jun;517:31-40. doi: 10.1016/j.cca.2021.02.004. Epub 2021 Feb 16. Clin Chim Acta. 2021. PMID: 33607071 Review.
-
Omentin roles in physiology and pathophysiology: an up-to-date comprehensive review.Arch Physiol Biochem. 2024 Dec;130(6):800-813. doi: 10.1080/13813455.2023.2283685. Epub 2023 Nov 23. Arch Physiol Biochem. 2024. PMID: 37994431 Review.
Cited by
-
Fat and inflammation: adipocyte-myeloid cell crosstalk in atherosclerosis.Front Immunol. 2023 Sep 15;14:1238664. doi: 10.3389/fimmu.2023.1238664. eCollection 2023. Front Immunol. 2023. PMID: 37781401 Free PMC article. Review.
-
Omentin-1 effects on mesenchymal stem cells: proliferation, apoptosis, and angiogenesis in vitro.Stem Cell Res Ther. 2017 Oct 10;8(1):224. doi: 10.1186/s13287-017-0676-1. Stem Cell Res Ther. 2017. PMID: 29017592 Free PMC article.
-
Decoding the transcriptome of calcified atherosclerotic plaque at single-cell resolution.Commun Biol. 2022 Oct 12;5(1):1084. doi: 10.1038/s42003-022-04056-7. Commun Biol. 2022. PMID: 36224302 Free PMC article.
-
Insights into the molecular mechanisms of diabetes-induced endothelial dysfunction: focus on oxidative stress and endothelial progenitor cells.Endocrine. 2015 Dec;50(3):537-67. doi: 10.1007/s12020-015-0709-4. Epub 2015 Aug 14. Endocrine. 2015. PMID: 26271514
-
Recent progress in adipocytokine research.Nagoya J Med Sci. 2023 Feb;85(1):23-26. doi: 10.18999/nagjms.85.1.23. Nagoya J Med Sci. 2023. PMID: 36923629 Free PMC article. No abstract available.
References
-
- Reilly M. P., Rader D. J. (2003) Circulation 108, 1546–1551 - PubMed
-
- Friedman J. M. (2003) Science 299, 856–858 - PubMed
-
- Yilmaz M. B., Biyikoglu S. F., Akin Y., Guray U., Kisacik H. L., Korkmaz S. (2003) Int. J. Obes. Relat. Metab. Disord. 27, 1541–1545 - PubMed
-
- Warley A., Powell J. M., Skepper J. N. (1995) Diabetologia 38, 413–421 - PubMed
-
- Lind L., Lithell H. (1993) Am. Heart J. 125, 1494–1497 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

