Ataxin-3 deubiquitination is coupled to Parkin ubiquitination via E2 ubiquitin-conjugating enzyme
- PMID: 22081612
- PMCID: PMC3249107
- DOI: 10.1074/jbc.M111.288449
Ataxin-3 deubiquitination is coupled to Parkin ubiquitination via E2 ubiquitin-conjugating enzyme
Abstract
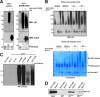
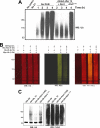
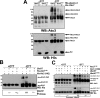
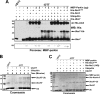
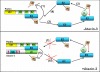
We reported previously that parkin, a Parkinson disease-associated E3 ubiquitin-ligase interacts with ataxin-3, a deubiquitinating enzyme associated with Machado-Joseph disease. Ataxin-3 was found to counteract parkin self-ubiquitination both in vitro and in cells. Moreover, ataxin-3-dependent deubiquitination of parkin required the catalytic cysteine 14 in ataxin-3, although the precise mechanism remained unclear. We report here that ataxin-3 interferes with the attachment of ubiquitin (Ub) onto parkin in real-time during conjugation but is unable to hydrolyze previously assembled parkin-Ub conjugates. The mechanism involves an ataxin-3-dependent stabilization of the complex between parkin and the E2 Ub-conjugating enzyme, which impedes the efficient charging of the E2 with Ub. Moreover, within this complex, the transfer of Ub from the E2 is diverted away from parkin and onto ataxin-3, further explaining how ataxin-3 deubiquitination is coupled to parkin ubiquitination. Taken together, our findings reveal an unexpected convergence upon the E2 Ub-conjugating enzyme in the regulation of an E3/deubiquitinating enzyme pair, with important implications for the function of parkin and ataxin-3, two proteins responsible for closely related neurodegenerative diseases.
Figures


References
-
- Kerscher O., Felberbaum R., Hochstrasser M. (2006) Annu. Rev. Cell Dev. Biol. 22, 159–180 - PubMed
-
- Pickart C. M., Eddins M. J. (2004) Biochim. Biophys. Acta 1695, 55–72 - PubMed
-
- Pelzer C., Kassner I., Matentzoglu K., Singh R. K., Wollscheid H. P., Scheffner M., Schmidtke G., Groettrup M. (2007) J. Biol. Chem. 282, 23010–23014 - PubMed
-
- Groettrup M., Pelzer C., Schmidtke G., Hofmann K. (2008) Trends Biochem. Sci. 33, 230–237 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

