Testosterone-induced relaxation of coronary arteries: activation of BKCa channels via the cGMP-dependent protein kinase
- PMID: 22081702
- PMCID: PMC3334243
- DOI: 10.1152/ajpheart.00046.2011
Testosterone-induced relaxation of coronary arteries: activation of BKCa channels via the cGMP-dependent protein kinase
Abstract
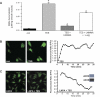
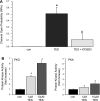
Androgens are reported to have both beneficial and detrimental effects on human cardiovascular health. The aim of this study was to characterize nongenomic signaling mechanisms in coronary artery smooth muscle (CASM) and define the ionic basis of testosterone (TES) action. TES-induced relaxation of endothelium-denuded porcine coronary arteries was nearly abolished by 20 nM iberiotoxin, a highly specific inhibitor of large-conductance, calcium-activated potassium (BK(Ca)) channels. Molecular patch-clamp studies confirmed that nanomolar concentrations of TES stimulated BK(Ca) channel activity by ∼100-fold and that inhibition of nitric oxide synthase (NOS) activity by N(G)-monomethyl-L-arginine nearly abolished this effect. Inhibition of nitric oxide (NO) synthesis or guanylyl cyclase activity also attenuated TES-induced coronary artery relaxation but did not alter relaxation due to 8-bromo-cGMP. Furthermore, we detected TES-stimulated NO production in porcine coronary arteries and in human CASM cells via stimulation of the type 1 neuronal NOS isoform. Inhibition of the cGMP-dependent protein kinase (PKG) attenuated TES-stimulated BK(Ca) channel activity, and direct assay determined that TES increased activity of PKG in a concentration-dependent fashion. Last, the stimulatory effect of TES on BK(Ca) channel activity was mimicked by addition of purified PKG to the cytoplasmic surface of a cell-free membrane patch from CASM myocytes (∼100-fold increase). These findings indicate that TES-induced relaxation of endothelium-denuded coronary arteries is mediated, at least in part, by enhanced NO production, leading to cGMP synthesis and PKG activation, which, in turn, opens BK(Ca) channels. These findings provide a molecular mechanism that could help explain why androgens have been reported to relax coronary arteries and relieve angina pectoris.
Figures

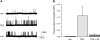



References
-
- Bowles DK, Maddali KK, Ganjam VK, Rubin LJ, Tharp DL, Turk JR, Heaps CL. Endogenous testosterone increases L-type Ca2+ channel expression in porcine coronary smooth muscle. Am J Physiol Heart Circ Physiol 287: H2091–H2098, 2004 - PubMed
-
- Cairrao E, Santos-Silva AJ, Verde I. PKG is involved in testosterone-induced vasorelaxation of human umbilical artery. Eur J Pharmacol 640: 94–101, 2010 - PubMed
-
- Ceballos G, Figueroa L, Rubio I, Gallo G, Garcia A, Martinez A, Yanez R, Perez J, Morato T, Chamorro G. Acute and nongenomic effects of testosterone on isolated and perfused rat heart. J Cardiovasc Pharmacol 33: 691–697, 1999 - PubMed
-
- Chou TM, Sudhir K, Hutchison SJ, Ko E, Amidon TM, Collins P, Chatterjee K. Testosterone induces dilation of canine coronary conductance and resistance arteries in vivo. Circulation 94: 2614–2619, 1996 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

