Computationally-guided optimization of a docking hit to yield catechol diethers as potent anti-HIV agents
- PMID: 22081993
- PMCID: PMC3258498
- DOI: 10.1021/jm201134m
Computationally-guided optimization of a docking hit to yield catechol diethers as potent anti-HIV agents
Abstract
A 5-μM docking hit has been optimized to an extraordinarily potent (55 pM) non-nucleoside inhibitor of HIV reverse transcriptase. Use of free energy perturbation (FEP) calculations to predict relative free energies of binding aided the optimizations by identifying optimal substitution patterns for phenyl rings and a linker. The most potent resultant catechol diethers feature terminal uracil and cyanovinylphenyl groups. A halogen bond with Pro95 likely contributes to the extreme potency of compound 42. In addition, several examples are provided illustrating failures of attempted grafting of a substructure from a very active compound onto a seemingly related scaffold to improve its activity.
Figures
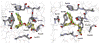








References
-
-
Kohlstaedt LA, Wang J, Friedman JM, Rice PA, Steitz TA. Crystal Structure at 3.5 Å Resolution of HIV-1 Reverse Transcriptase Complexed with an Inhibitor. Science. 1992;256:1783–1790.. For a review, see: Prajapati DG, Ramajayam R, Yadav MR, Giridhar R. The search for potent, small molecule NNRTIs: A review. Bioorg Med Chem. 2009;17:5744–5762.
-
-
- Adams J, Patel N, Mankaryous N, Tadros M, Miller CD. HIV/AIDS: Nonnucleoside Reverse Transcriptase Inhibitor Resistance and the Role of the Second-Generation Agents. Ann Pharmacotherapy. 2010;44:157–165. - PubMed
- Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The Challenge of Finding a Cure for HIV Infection. Science. 2009;323:1304–1307. - PubMed
-
-
For a review, see: Jorgensen WL. Efficient Drug Lead Discovery and Optimization. Acc Chem Res. 2009;42:724–733.
-
-
- Zeevaart JG, Wang L, Thakur VV, Leung CS, Tirado-Rives J, Bailey CM, Domaoal RA, Anderson KS, Jorgensen WL. Optimization of Azoles as Anti-HIV Agents Guided by Free-Energy Calculations. J Am Chem Soc. 2008;130:9492–9499. - PMC - PubMed
- Leung CS, Zeevaart JG, Domaoal RA, Bollini M, Thakur VV, Spasov K, Anderson KS, Jorgensen WL. Eastern extension of azoles as non-nucleoside inhibitors of HIV-1 reverse transcriptase; cyano group alternatives. Bioorg Med Chem Lett. 2010;20:2485–2488. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

