Influenza H1N1 (swine flu) vaccination: a safety surveillance feasibility study using self-reporting of serious adverse events and pregnancy outcomes
- PMID: 22082196
- PMCID: PMC3403208
- DOI: 10.1111/j.1365-2125.2011.04142.x
Influenza H1N1 (swine flu) vaccination: a safety surveillance feasibility study using self-reporting of serious adverse events and pregnancy outcomes
Abstract
Aims: During the global H1N1 influenza A (swine flu) pandemic 2009-2010, swine flu vaccines were expeditiously licensed and a mass vaccination programme for high risk groups, including pregnant women, was introduced in the UK. This pilot active safety surveillance study was performed to establish the feasibility of rapidly monitoring the new swine flu vaccines in large patient numbers receiving or offered the vaccination under normal conditions of use within a short time frame.
Methods: A cohort design with safety data capture through modern technologies was carried out in Scotland, UK during the winter swine flu vaccination programme 2009-2010 in individuals receiving or offered the swine flu vaccination. The main outcome measures were self-reported serious adverse events (SAEs) and pregnancy outcomes.
Results: The cohort comprised 4066 people; 3754 vaccinated and 312 offered the vaccination but not vaccinated. There were 939 self-reported events (838 different events), 53 judged to fit SAE criteria by the investigators, with nine judged as possibly, probably or definitely vaccine related. None of the seven deaths (six in vaccinees) were judged as vaccine related. One hundred and twenty-eight women reported 130 pregnancies during the study with 117 pregnant at study start. There were reports of four miscarriages in three women and six possible congenital abnormalities in live births.
Conclusions: Overall, no significant safety issues were identified. The methodology and use of modern technologies to collect safety data from large numbers of patients was successful and could be used again in similar safety studies.
© 2011 The Authors. British Journal of Clinical Pharmacology © 2011 The British Pharmacological Society.
Figures
References
-
- Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, Gubareva LV, Xu X, Bridges CB, Uyeki TM. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–15. - PubMed
-
- The Scottish Government. Swine flu pandemic [online] 2011. Available at http://www.scotland.gov.uk/Topics/Health/health/flu/swineflu (last accessed 13 April 2011). The Scottish Government 2011 March 1.
-
- MHRA. Final Public Summary. UK suspected adverse reaction analysis. Swine Flu (H1N1) Vaccines – Celvapan and Pandemrix. [online] 2010. Available at http://www.mhra.gov.uk/home/groups/pl-p/documents/websiteresources/con07... (last accessed 10 May 2011)
-
- Greenberg ME, Lai MH, Hartel GF, Wichems CH, Gittleson C, Bennet J, Dawson G, Hu W, Leggio C, Washington D, Basser RL. Response to a monovalent 2009 influenza A (H1N1) vaccine. N Engl J Med. 2009;361:2405–13. - PubMed
-
- Plennevaux E, Sheldon E, Blatter M, Reeves-Hoche MK, Denis M. Immune response after a single vaccination against 2009 influenza A H1N1 in USA: a preliminary report of two randomised controlled phase 2 trials. Lancet. 2010;375:41–8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

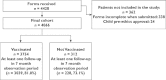

 ); declined H1N1 vaccine (
); declined H1N1 vaccine ( )
)