Hyperpolarizing GABAergic transmission depends on KCC2 function and membrane potential
- PMID: 22082832
- PMCID: PMC3265795
- DOI: 10.4161/chan.5.6.17952
Hyperpolarizing GABAergic transmission depends on KCC2 function and membrane potential
Abstract
KCC2 comprises the major Cl(-) extruding mechanism in most adult neurons. Hyperpolarizing GABAergic transmission depends on KCC2 function. We recently demonstrated that glutamate reduces KCC2 function by a phosphorylation-dependent mechanism that leads to excitatory GABA responses. Here we investigated the methods by which to estimate changes in E(GABA), as well as the processes that lead to depolarizing GABA responses and their effects on neuronal excitability. We demonstrated that current-clamp recordings of membrane potential responses to GABA can determine upper and lower limits of E(GABA). We also further characterized depolarizing GABA responses, which both excited and inhibited neurons. Our analyses revealed that persistently active GABA(A) receptors contributed to loading Cl(-) during the glutamate exposure, indicating that tonic inhibition can facilitate the development of depolarizing GABA responses and increase excitability after pathophysiological insults. Finally, we demonstrated that hyperpolarizing GABA responses could temporarily switch to depolarizing responses when they coincided with an afterhyperpolarization.
Figures
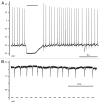
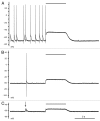
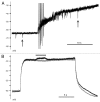
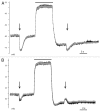
Comment on
-
NMDA receptor activity downregulates KCC2 resulting in depolarizing GABAA receptor-mediated currents.Nat Neurosci. 2011 Jun;14(6):736-43. doi: 10.1038/nn.2806. Epub 2011 May 1. Nat Neurosci. 2011. PMID: 21532577 Free PMC article.
Similar articles
-
Could tuning of the inhibitory tone involve graded changes in neuronal chloride transport?Neuropharmacology. 2015 Aug;95:321-31. doi: 10.1016/j.neuropharm.2015.03.026. Epub 2015 Apr 3. Neuropharmacology. 2015. PMID: 25843644
-
Evidence for Two Subpopulations of Cerebrospinal Fluid-Contacting Neurons with Opposite GABAergic Signaling in Adult Mouse Spinal Cord.J Neurosci. 2024 May 29;44(22):e2289222024. doi: 10.1523/JNEUROSCI.2289-22.2024. J Neurosci. 2024. PMID: 38684364 Free PMC article.
-
An evolutionarily conserved switch in response to GABA affects development and behavior of the locomotor circuit of Caenorhabditis elegans.Genetics. 2015 Apr;199(4):1159-72. doi: 10.1534/genetics.114.173963. Epub 2015 Feb 2. Genetics. 2015. PMID: 25644702 Free PMC article.
-
Role of activity-dependent regulation of neuronal chloride homeostasis in development.Curr Opin Neurobiol. 2007 Feb;17(1):81-6. doi: 10.1016/j.conb.2007.01.002. Epub 2007 Jan 17. Curr Opin Neurobiol. 2007. PMID: 17234400 Review.
-
Double-edged GABAergic synaptic transmission in seizures: The importance of chloride plasticity.Brain Res. 2018 Dec 15;1701:126-136. doi: 10.1016/j.brainres.2018.09.008. Epub 2018 Sep 7. Brain Res. 2018. PMID: 30201259 Review.
Cited by
-
Direct activation of KCC2 arrests benzodiazepine refractory status epilepticus and limits the subsequent neuronal injury in mice.Cell Rep Med. 2023 Mar 21;4(3):100957. doi: 10.1016/j.xcrm.2023.100957. Epub 2023 Mar 7. Cell Rep Med. 2023. PMID: 36889319 Free PMC article.
-
The Therapeutic Potential of Neuronal K-Cl Co-Transporter KCC2 in Huntington's Disease and Its Comorbidities.Int J Mol Sci. 2020 Nov 30;21(23):9142. doi: 10.3390/ijms21239142. Int J Mol Sci. 2020. PMID: 33266310 Free PMC article. Review.
-
Inhibiting with-no-lysine kinases enhances K+/Cl- cotransporter 2 activity and limits status epilepticus.Brain. 2022 Apr 29;145(3):950-963. doi: 10.1093/brain/awab343. Brain. 2022. PMID: 34528073 Free PMC article.
-
Homeostatic regulation of KCC2 activity by the zinc receptor mZnR/GPR39 during seizures.Neurobiol Dis. 2015 Sep;81:4-13. doi: 10.1016/j.nbd.2014.12.020. Epub 2015 Jan 3. Neurobiol Dis. 2015. PMID: 25562657 Free PMC article.
-
Disrupted Cl(-) homeostasis contributes to reductions in the inhibitory efficacy of diazepam during hyperexcited states.Eur J Neurosci. 2013 Aug;38(3):2453-67. doi: 10.1111/ejn.12241. Epub 2013 Apr 29. Eur J Neurosci. 2013. PMID: 23627375 Free PMC article.
References
-
- Payne JA, Stevenson TJ, Donaldson LF. Molecular characterization of a putative K-Cl cotransporter in rat brain. A neuronal-specific isoform. J Biol Chem. 1996;271:16245–16252. - PubMed
-
- Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. - PubMed
-
- Plotkin MD, Snyder EY, Hebert SC, Delpire E. Expression of the Na-K-2Cl cotransporter is developmentally regulated in postnatal rat brains: a possible mechanism underlying GABA's excitatory role in immature brain. J Neurobiol. 1997;33:781–795. - PubMed
-
- Blaesse P, Airaksinen MS, Rivera C, Kaila K. Cation-chloride cotransporters and neuronal function. Neuron. 2009;61:820–838. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
