Interactions between gaze-evoked blinks and gaze shifts in monkeys
- PMID: 22083094
- PMCID: PMC3262917
- DOI: 10.1007/s00221-011-2937-z
Interactions between gaze-evoked blinks and gaze shifts in monkeys
Abstract
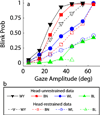
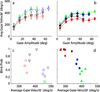
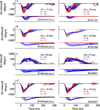
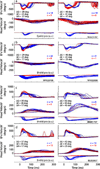
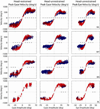
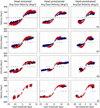
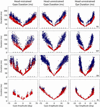
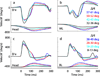
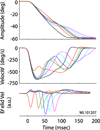
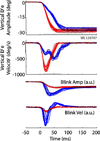
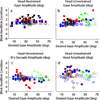
Rapid eyelid closure, or a blink, often accompanies head-restrained and head-unrestrained gaze shifts. This study examines the interactions between such gaze-evoked blinks and gaze shifts in monkeys. Blink probability increases with gaze amplitude and at a faster rate for head-unrestrained movements. Across animals, blink likelihood is inversely correlated with the average gaze velocity of large-amplitude control movements. Gaze-evoked blinks induce robust perturbations in eye velocity. Peak and average velocities are reduced, duration is increased, but accuracy is preserved. The temporal features of the perturbation depend on factors such as the time of blink relative to gaze onset, inherent velocity kinematics of control movements, and perhaps initial eye-in-head position. Although variable across animals, the initial effect is a reduction in eye velocity, followed by a reacceleration that yields two or more peaks in its waveform. Interestingly, head velocity is not attenuated; instead, it peaks slightly later and with a larger magnitude. Gaze latency is slightly reduced on trials with gaze-evoked blinks, although the effect was more variable during head-unrestrained movements; no reduction in head latency is observed. Preliminary data also demonstrate a similar perturbation of gaze-evoked blinks during vertical saccades. The results are compared with previously reported effects of reflexive blinks (evoked by air-puff delivered to one eye or supraorbital nerve stimulation) and discussed in terms of effects of blinks on saccadic suppression, neural correlates of the altered eye velocity signals, and implications on the hypothesis that the attenuation in eye velocity is produced by a head movement command.
Figures


Similar articles
-
Blink-perturbed saccades in monkey. I. Behavioral analysis.J Neurophysiol. 2000 Jun;83(6):3411-29. doi: 10.1152/jn.2000.83.6.3411. J Neurophysiol. 2000. PMID: 10848559
-
Combined eye-head gaze shifts produced by electrical stimulation of the superior colliculus in rhesus monkeys.J Neurophysiol. 1996 Aug;76(2):927-52. doi: 10.1152/jn.1996.76.2.927. J Neurophysiol. 1996. PMID: 8871209
-
Temporal interactions of air-puff-evoked blinks and saccadic eye movements: insights into motor preparation.J Neurophysiol. 2005 Mar;93(3):1718-29. doi: 10.1152/jn.00854.2004. Epub 2004 Oct 6. J Neurophysiol. 2005. PMID: 15469959 Free PMC article.
-
Not looking while leaping: the linkage of blinking and saccadic gaze shifts.Exp Brain Res. 1994;100(2):337-44. doi: 10.1007/BF00227203. Exp Brain Res. 1994. PMID: 7813670
-
Coordination of the eyes and head during visual orienting.Exp Brain Res. 2008 Oct;190(4):369-87. doi: 10.1007/s00221-008-1504-8. Epub 2008 Aug 13. Exp Brain Res. 2008. PMID: 18704387 Free PMC article. Review.
Cited by
-
Phylogenetically-controlled correlates of primate blinking behaviour.PeerJ. 2021 Feb 17;9:e10950. doi: 10.7717/peerj.10950. eCollection 2021. PeerJ. 2021. PMID: 33643718 Free PMC article.
-
Target Displacements during Eye Blinks Trigger Automatic Recalibration of Gaze Direction.Curr Biol. 2017 Feb 6;27(3):445-450. doi: 10.1016/j.cub.2016.12.029. Epub 2017 Jan 19. Curr Biol. 2017. PMID: 28111150 Free PMC article.
-
Hierarchical control of two-dimensional gaze saccades.J Comput Neurosci. 2014 Jun;36(3):355-82. doi: 10.1007/s10827-013-0477-1. Epub 2013 Sep 6. J Comput Neurosci. 2014. PMID: 24062206 Free PMC article.
-
Neural encoding of instantaneous kinematics of eye-head gaze shifts in monkey superior Colliculus.Commun Biol. 2023 Sep 9;6(1):927. doi: 10.1038/s42003-023-05305-z. Commun Biol. 2023. PMID: 37689726 Free PMC article.
-
Fast gaze reorientations by combined movements of the eye, head, trunk and lower extremities.Exp Brain Res. 2015 May;233(5):1639-50. doi: 10.1007/s00221-015-4238-4. Epub 2015 Mar 12. Exp Brain Res. 2015. PMID: 25761968 Free PMC article.
References
-
- Anastasopoulos D, Ziavra N, Hollands M, Bronstein A. Gaze displacement and inter-segmental coordination during large whole body voluntary rotations. Exp Brain Res. 2009;193:323–336. - PubMed
-
- Anderson RW, Keller EL, Gandhi NJ, Das S. Two dimensional saccade-related population activity in superior colliculus in monkey. J Neurophysiol. 1998;80:798–817. - PubMed
-
- Bechara BP, Gandhi NJ. Effect of blinks on the relationship between eye velocity and high-frequency burst neurons in the pons. Soc Neurosci Abstr Program No. 894.4. 2010a
-
- Becker W, Fuchs AF. Lid-eye coordination during vertical gaze changes in man and monkey. J Neurophysiol. 1988;60:1227–1252. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

