PD 404,182 is a virocidal small molecule that disrupts hepatitis C virus and human immunodeficiency virus
- PMID: 22083468
- PMCID: PMC3264232
- DOI: 10.1128/AAC.05722-11
PD 404,182 is a virocidal small molecule that disrupts hepatitis C virus and human immunodeficiency virus
Abstract
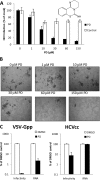
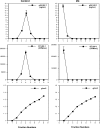
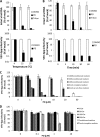
We describe a virucidal small molecule, PD 404,182, that is effective against hepatitis C virus (HCV) and human immunodeficiency virus (HIV). The median 50% inhibitory concentrations (IC(50)s) for the antiviral effect of PD 404,182 against HCV and HIV in cell culture are 11 and 1 μM, respectively. The antiviral activity of PD 404,182 is due to the physical disruption of virions that is accompanied to various degrees (depending on the virus and exposure temperature/time) by the release of viral nucleic acids into the surrounding medium. PD 404,182 does not directly lyse liposomal membranes even after extended exposure, and it shows no attenuation in antiviral activity when preincubated with liposomes of various lipid compositions, suggesting that the compound inactivates viruses through interaction with a nonlipid structural component of the virus. The virucidal activity of PD 404,182 appears to be virus specific, as little to no viral inactivation was detected with the enveloped Dengue and Sindbis viruses. PD 404,182 effectively inactivates a broad range of primary isolates of HIV-1 as well as HIV-2 and simian immunodeficiency virus (SIV), and it does not exhibit significant cytotoxicity with multiple human cell lines in vitro (50% cytotoxic concentration, >300 μM). The compound is fully active in cervical fluids, although it exhibits decreased potency in the presence of human serum, retains its full antiviral potency for 8 h when in contact with cells, and is effective against both cell-free and cell-associated HIV. These qualities make PD 404,182 an attractive candidate anti-HIV microbicide for the prevention of HIV transmission through sexual intercourse.
Figures
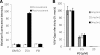

Similar articles
-
Structural and Functional Characterization of Membrane Fusion Inhibitors with Extremely Potent Activity against Human Immunodeficiency Virus Type 1 (HIV-1), HIV-2, and Simian Immunodeficiency Virus.J Virol. 2018 Sep 26;92(20):e01088-18. doi: 10.1128/JVI.01088-18. Print 2018 Oct 15. J Virol. 2018. PMID: 30089693 Free PMC article.
-
Entry of hepatitis C virus and human immunodeficiency virus is selectively inhibited by carbohydrate-binding agents but not by polyanions.Virology. 2007 Sep 15;366(1):40-50. doi: 10.1016/j.virol.2007.04.008. Epub 2007 May 11. Virology. 2007. PMID: 17498767
-
Evaluation of PD 404,182 as an anti-HIV and anti-herpes simplex virus microbicide.Antimicrob Agents Chemother. 2014;58(2):687-97. doi: 10.1128/AAC.02000-13. Epub 2013 Nov 11. Antimicrob Agents Chemother. 2014. PMID: 24217696 Free PMC article.
-
Inactivation of enveloped viruses in human bodily fluids by purified lipids.Ann N Y Acad Sci. 1994 Jun 6;724:457-64. doi: 10.1111/j.1749-6632.1994.tb38947.x. Ann N Y Acad Sci. 1994. PMID: 8030973 Review.
-
Virus attachment and entry offer numerous targets for antiviral therapy.Curr Pharm Des. 2004;10(30):3701-12. doi: 10.2174/1381612043382729. Curr Pharm Des. 2004. PMID: 15579065 Review.
Cited by
-
Graphene quantum dots as potential broad-spectrum antiviral agents.Nanoscale Adv. 2025 Jan 30;7(7):2032-2038. doi: 10.1039/d4na00879k. eCollection 2025 Mar 25. Nanoscale Adv. 2025. PMID: 39974343 Free PMC article.
-
Identification of 3,4-Dihydro-2H,6H-pyrimido[1,2-c][1,3]benzothiazin-6-imine Derivatives as Novel Selective Inhibitors of Plasmodium falciparum Dihydroorotate Dehydrogenase.Int J Mol Sci. 2021 Jul 5;22(13):7236. doi: 10.3390/ijms22137236. Int J Mol Sci. 2021. PMID: 34281290 Free PMC article.
-
CuI nanoparticle-catalyzed synthesis of tetracyclic benzo[e]benzo[4,5]imidazo[1,2-c][1,3]thiazin-6-imine heterocycles by SNAr-type C-S, C-N bond formation from isothiocyanatobenzenes and benzimidazoles.RSC Adv. 2018 Jun 19;8(39):22259-22267. doi: 10.1039/c8ra02552e. eCollection 2018 Jun 13. RSC Adv. 2018. PMID: 35541714 Free PMC article.
-
Synthesis of imidazo[4,5-e][1,3]thiazino[2,3-c][1,2,4]triazines via a base-induced rearrangement of functionalized imidazo[4,5-e]thiazolo[2,3-c][1,2,4]triazines.Beilstein J Org Chem. 2023 Jul 28;19:1047-1054. doi: 10.3762/bjoc.19.80. eCollection 2023. Beilstein J Org Chem. 2023. PMID: 37533878 Free PMC article.
-
PA-824 inhibits porcine epidemic diarrhea virus infection in vivo and in vitro by inhibiting p53 activation.J Virol. 2024 Jul 23;98(7):e0041323. doi: 10.1128/jvi.00413-23. Epub 2024 Jun 12. J Virol. 2024. PMID: 38864728 Free PMC article.
References
-
- Birck MR, Holler TP, Woodard RW. 2000. Identification of a slow tight-binding inhibitor of 3-deoxy-D-manno-octulosonic acid 8-phosphate synthase. J. Am. Chem. Soc. 122:9334–9335
-
- Bremer CM, Bung C, Kott N, Hardt M, Glebe D. 2009. Hepatitis B virus infection is dependent on cholesterol in the viral envelope. Cell Microbiol. 11:249–260 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

