Immunobiotic Lactobacillus jensenii elicits anti-inflammatory activity in porcine intestinal epithelial cells by modulating negative regulators of the Toll-like receptor signaling pathway
- PMID: 22083706
- PMCID: PMC3255675
- DOI: 10.1128/IAI.05729-11
Immunobiotic Lactobacillus jensenii elicits anti-inflammatory activity in porcine intestinal epithelial cells by modulating negative regulators of the Toll-like receptor signaling pathway
Abstract
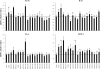
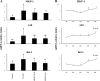
The effect of Lactobacillus jensenii TL2937 on the inflammatory immune response triggered by enterotoxigenic Escherichia coli (ETEC) and lipopolysaccharide (LPS) in a porcine intestinal epitheliocyte cell line (PIE cells) was evaluated. Challenges with ETEC or LPS elicited Toll-like receptor 4 (TLR4)-mediated inflammatory responses in cultured PIE cells, indicating that our cell line may be useful for studying inflammation in the guts of weaning piglets. In addition, we demonstrated that L. jensenii TL2937 attenuated the expression of proinflammatory cytokines and chemokines caused by ETEC or LPS challenge by downregulating TLR4-dependent nuclear factorκB (NF-κB) and mitogen-activated protein kinase (MAPK) activation. Furthermore, we demonstrated that L. jensenii TL2937 stimulation of PIE cells upregulated three negative regulators of TLRs: A20, Bcl-3, and MKP-1, deepening the understanding of an immunobiotic mechanism of action. L. jensenii TL2937-mediated induction of negative regulators of TLRs would have a substantial physiological impact on homeostasis in PIE cells, because excessive TLR inflammatory signaling would be downregulated. These results indicated that PIE cells can be used to study the mechanisms involved in the protective activity of immunobiotics against intestinal inflammatory damage and may provide useful information for the development of new immunologically functional feeds that help to prevent inflammatory intestinal disorders, including weaning-associated intestinal inflammation.
Figures





References
-
- Abreu MT, Fukata M, Arditi M. 2005. TLR signaling in the gut in health and disease. J. Immunol. 174: 4453–4460 - PubMed
-
- Alvarez S, Villena J, Salva S. 2009. Humoral immunity against respiratory pathogens: can lactic acid bacteria improve it? Res. Adv. Infect. Immun. 1: 1–9
-
- Alvarez S, Villena J, Tohno M, Salva S, Kitazawa H. 2009. Modulation of innate immunity by lactic acid bacteria: impact on host response to infections. Curr. Res. Immunol. 3: 87–126
-
- Biswas SK, Lopez-Collazo E. 2009. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 30: 475–487 - PubMed
-
- Boone DL, et al. 2004. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat. Immunol. 5: 1052–1060 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Miscellaneous

