The repeat-in-toxin family member TosA mediates adherence of uropathogenic Escherichia coli and survival during bacteremia
- PMID: 22083710
- PMCID: PMC3264304
- DOI: 10.1128/IAI.05713-11
The repeat-in-toxin family member TosA mediates adherence of uropathogenic Escherichia coli and survival during bacteremia
Abstract
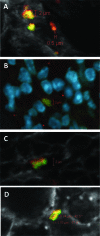
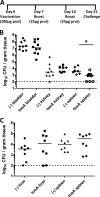
Uropathogenic Escherichia coli (UPEC) is responsible for the majority of uncomplicated urinary tract infections (UTI) and represents the most common bacterial infection in adults. UPEC utilizes a wide range of virulence factors to colonize the host, including the novel repeat-in-toxin (RTX) protein TosA, which is specifically expressed in the host urinary tract and contributes significantly to the virulence and survival of UPEC. tosA, found in strains within the B2 phylogenetic subgroup of E. coli, serves as a marker for strains that also contain a large number of well-characterized UPEC virulence factors. The presence of tosA in an E. coli isolate predicts successful colonization of the murine model of ascending UTI, regardless of the source of the isolate. Here, a detailed analysis of the function of tosA revealed that this gene is transcriptionally linked to genes encoding a conserved type 1 secretion system similar to other RTX family members. TosA localized to the cell surface and was found to mediate (i) adherence to host cells derived from the upper urinary tract and (ii) survival in disseminated infections and (iii) to enhance lethality during sepsis (as assessed in two different animal models of infection). An experimental vaccine, using purified TosA, protected vaccinated animals against urosepsis. From this work, it was concluded that TosA belongs to a novel group of RTX proteins that mediate adherence and host damage during UTI and urosepsis and could be a novel target for the development of therapeutics to treat ascending UTIs.
Figures







References
-
- Brooks HJ, O'Grady F, McSherry MA, Cattell WR. 1980. Uropathogenic properties of Escherichia coli in recurrent urinary-tract infection. J. Med. Microbiol. 13:57–68 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

