Increase in dNTP pool size during the DNA damage response plays a key role in spontaneous and induced-mutagenesis in Escherichia coli
- PMID: 22084087
- PMCID: PMC3228436
- DOI: 10.1073/pnas.1113664108
Increase in dNTP pool size during the DNA damage response plays a key role in spontaneous and induced-mutagenesis in Escherichia coli
Abstract
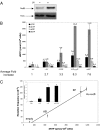
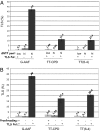
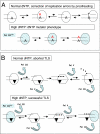
Exposure of Escherichia coli to UV light increases expression of NrdAB, the major ribonucleotide reductase leading to a moderate increase in dNTP levels. The role of elevated dNTP levels during translesion synthesis (TLS) across specific replication-blocking lesions was investigated. Here we show that although the specialized DNA polymerase PolV is necessary for replication across UV-lesions, such as cyclobutane pyrimidine dimers or pyrimidine(6-4)pyrimidone photoproduct, Pol V per se is not sufficient. Indeed, efficient TLS additionally requires elevated dNTP levels. Similarly, for the bypass of an N-2-acetylaminofluorene-guanine adduct that requires Pol II instead of PolV, efficient TLS is only observed under conditions of high dNTP levels. We suggest that increased dNTP levels transiently modify the activity balance of Pol III (i.e., increasing the polymerase and reducing the proofreading functions). Indeed, we show that the stimulation of TLS by elevated dNTP levels can be mimicked by genetic inactivation of the proofreading function (mutD5 allele). We also show that spontaneous mutagenesis increases proportionally to dNTP pool levels, thus defining a unique spontaneous mutator phenotype. The so-called "dNTP mutator" phenotype does not depend upon any of the specialized DNA polymerases, and is thus likely to reflect an increase in Pol III's own replication errors because of the modified activity balance of Pol III. As up-regulation of the dNTP pool size represents a common physiological response to DNA damage, the present model is likely to represent a general and unique paradigm for TLS pathways in many organisms.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Nordlund P, Reichard P. Ribonucleotide reductases. Annu Rev Biochem. 2006;75:681–706. - PubMed
-
- Kunz BA, et al. International Commission for Protection Against Environmental Mutagens and Carcinogens. Deoxyribonucleoside triphosphate levels: A critical factor in the maintenance of genetic stability. Mutat Res. 1994;318:1–64. - PubMed
-
- Chabes A, et al. Survival of DNA damage in yeast directly depends on increased dNTP levels allowed by relaxed feedback inhibition of ribonucleotide reductase. Cell. 2003;112:391–401. - PubMed
-
- Wheeler LJ, Rajagopal I, Mathews CK. Stimulation of mutagenesis by proportional deoxyribonucleoside triphosphate accumulation in Escherichia coli. DNA Repair (Amst) 2005;4:1450–1456. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

