Glucocorticoid therapy of antigen-induced arthritis depends on the dimerized glucocorticoid receptor in T cells
- PMID: 22084093
- PMCID: PMC3228447
- DOI: 10.1073/pnas.1105857108
Glucocorticoid therapy of antigen-induced arthritis depends on the dimerized glucocorticoid receptor in T cells
Abstract
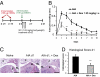
Despite several side effects, glucocorticoids (GCs) have been widely used for 60 y to treat rheumatoid arthritis on the basis of their antiinflammatory effects. However, the cells targeted by GCs and the transcriptional mechanisms underlying their actions through the glucocorticoid receptor (GR) in steroid therapy remain poorly defined. Using cell type-specific GR-deficient mice subjected to antigen-induced arthritis (AIA) as a model of human rheumatoid arthritis, we show that GC action on T cells but not myeloid cells is critical for therapeutic intervention in AIA. Furthermore, the resistance of mice expressing a DNA binding-defective GR (GR(dim)) to GC treatment reveals that dimerization of the GR is indispensable for the antiinflammatory effects. In these mice, the GC-induced suppression of T(H)1 and T(H)17 cell-derived proinflammatory cytokines is impaired. Our finding that IL-17A(-/-) mice are resistant to GC therapy, whereas IFN-γ(-/-) mice respond as efficiently as WT mice implies that IL-17-producing T cells and not IFN-γ-producing T cells are the most important targets for an efficient GC therapy. The present study's identification of the critical cell type and the mode of GR action in steroid therapy of AIA significantly advances our understanding of steroid therapy and should lead to therapies with greater efficiency and fewer side effects.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
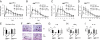


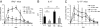
References
-
- Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85:307–310. - PubMed
-
- Hench PS, Kendall EC, Slocumb CH, Polley HF. Effects of cortisone acetate and pituitray ACTH on rheumatoid arthritis, rheumatic fever and certain other conditions. Arch Intern Med. 1950;85:545–566. - PubMed
-
- Kirwan J, Power L. Glucocorticoids: Action and new therapeutic insights in rheumatoid arthritis. Curr Opin Rheumatol. 2007;19:233–237. - PubMed
-
- Baschant U, Tuckermann J. The role of the glucocorticoid receptor in inflammation and immunity. J Steroid Biochem Mol Biol. 2010;120:69–75. - PubMed
-
- Tuckermann JP, Kleiman A, McPherson KG, Reichardt HM. Molecular mechanisms of glucocorticoids in the control of inflammation and lymphocyte apoptosis. Crit Rev Clin Lab Sci. 2005;42:71–104. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

