Wnt signaling and a Smad pathway blockade direct the differentiation of human pluripotent stem cells to multipotent neural crest cells
- PMID: 22084120
- PMCID: PMC3228464
- DOI: 10.1073/pnas.1113746108
Wnt signaling and a Smad pathway blockade direct the differentiation of human pluripotent stem cells to multipotent neural crest cells
Erratum in
- Proc Natl Acad Sci U S A. 2012 Jun 5;109(23):9220
Abstract
Neural crest stem cells can be isolated from differentiated cultures of human pluripotent stem cells, but the process is inefficient and requires cell sorting to obtain a highly enriched population. No specific method for directed differentiation of human pluripotent cells toward neural crest stem cells has yet been reported. This severely restricts the utility of these cells as a model for disease and development and for more applied purposes such as cell therapy and tissue engineering. In this report, we use small-molecule compounds in a single-step method for the efficient generation of self-renewing neural crest-like stem cells in chemically defined media. This approach is accomplished directly from human pluripotent cells without the need for coculture on feeder layers or cell sorting to obtain a highly enriched population. Critical to this approach is the activation of canonical Wnt signaling and concurrent suppression of the Activin A/Nodal pathway. Over 12-14 d, pluripotent cells are efficiently specified along the neuroectoderm lineage toward p75(+) Hnk1(+) Ap2(+) neural crest-like cells with little or no contamination by Pax6(+) neural progenitors. This cell population can be clonally amplified and maintained for >25 passages (>100 d) while retaining the capacity to differentiate into peripheral neurons, smooth muscle cells, and mesenchymal precursor cells. Neural crest-like stem cell-derived mesenchymal precursors have the capacity for differentiation into osteocytes, chondrocytes, and adipocytes. In sum, we have developed methods for the efficient generation of self-renewing neural crest stem cells that greatly enhance their potential utility in disease modeling and regenerative medicine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

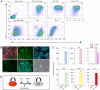



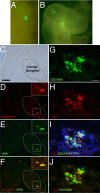
References
-
- Meulemans D, Bronner-Fraser M. Gene-regulatory interactions in neural crest evolution and development. Dev Cell. 2004;7(3):291–299. - PubMed
-
- García-Castro MI, Marcelle C, Bronner-Fraser M. Ectodermal Wnt function as a neural crest inducer. Science. 2002;297:848–851. - PubMed
-
- Patthey C, Edlund T, Gunhaga L. Wnt-regulated temporal control of BMP exposure directs the choice between neural plate border and epidermal fate. Development. 2009;136(1):73–83. - PubMed
-
- Wilson SI, et al. The status of Wnt signalling regulates neural and epidermal fates in the chick embryo. Nature. 2001;411:325–330. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

