The secret life of α-catenin: moonlighting in morphogenesis
- PMID: 22084304
- PMCID: PMC3257527
- DOI: 10.1083/jcb.201103106
The secret life of α-catenin: moonlighting in morphogenesis
Abstract
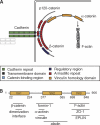
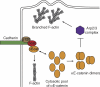
Cadherin-based intercellular adhesions are important determinants of proper tissue architecture. These adhesions must be both stable and dynamic to maintain tissue integrity as cells undergo morphogenetic movements during development. The role of α-catenin in this process has been vigorously debated due to conflicting in vitro and in vivo evidence regarding its molecular mechanism of action. Recent data supports the classical view that α-catenin facilitates actin attachments at adherens junctions, but also suggests that α-catenin may act as a force transducer, and may have additional roles in the cytoplasm. These multiple functions for α-catenin converge on the regulation of adhesion and may help to explain its stable yet dynamic nature.
© 2011 Maiden and Hardin
Figures

References
-
- Aberle H., Butz S., Stappert J., Weissig H., Kemler R., Hoschuetzky H. 1994. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J. Cell Sci. 107:3655–3663 - PubMed
-
- Barstead R.J., Waterston R.H. 1989. The basal component of the nematode dense-body is vinculin. J. Biol. Chem. 264:10177–10185 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

