A quantitative model for cyclin-dependent kinase control of the cell cycle: revisited
- PMID: 22084384
- PMCID: PMC3203462
- DOI: 10.1098/rstb.2011.0082
A quantitative model for cyclin-dependent kinase control of the cell cycle: revisited
Abstract
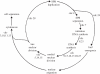
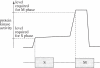
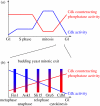
The eukaryotic cell division cycle encompasses an ordered series of events. Chromosomal DNA is replicated during S phase of the cell cycle before being distributed to daughter cells in mitosis. Both S phase and mitosis in turn consist of an intricately ordered sequence of molecular events. How cell cycle ordering is achieved, to promote healthy cell proliferation and avert insults on genomic integrity, has been a theme of Paul Nurse's research. To explain a key aspect of cell cycle ordering, sequential S phase and mitosis, Stern & Nurse proposed 'A quantitative model for cdc2 control of S phase and mitosis in fission yeast'. In this model, S phase and mitosis are ordered by their dependence on increasing levels of cyclin-dependent kinase (Cdk) activity. Alternative mechanisms for ordering have been proposed that rely on checkpoint controls or on sequential waves of cyclins with distinct substrate specificities. Here, we review these ideas in the light of experimental evidence that has meanwhile accumulated. Quantitative Cdk control emerges as the basis for cell cycle ordering, fine-tuned by cyclin specificity and checkpoints. We propose a molecular explanation for quantitative Cdk control, based on thresholds imposed by Cdk-counteracting phosphatases, and discuss its implications.
Figures

Similar articles
-
Differential susceptibility of yeast S and M phase CDK complexes to inhibitory tyrosine phosphorylation.Curr Biol. 2007 Jul 17;17(14):1181-9. doi: 10.1016/j.cub.2007.05.075. Epub 2007 Jul 5. Curr Biol. 2007. PMID: 17614281 Free PMC article.
-
Cyclin-Specific Docking Mechanisms Reveal the Complexity of M-CDK Function in the Cell Cycle.Mol Cell. 2019 Jul 11;75(1):76-89.e3. doi: 10.1016/j.molcel.2019.04.026. Epub 2019 May 14. Mol Cell. 2019. PMID: 31101497 Free PMC article.
-
A quantitative model for ordered Cdk substrate dephosphorylation during mitotic exit.Cell. 2011 Nov 11;147(4):803-14. doi: 10.1016/j.cell.2011.09.047. Cell. 2011. PMID: 22078879
-
[Molecular mechanisms controlling the cell cycle: fundamental aspects and implications for oncology].Cancer Radiother. 2001 Apr;5(2):109-29. doi: 10.1016/s1278-3218(01)00087-7. Cancer Radiother. 2001. PMID: 11355576 Review. French.
-
The Multiple Roles of the Cdc14 Phosphatase in Cell Cycle Control.Int J Mol Sci. 2020 Jan 21;21(3):709. doi: 10.3390/ijms21030709. Int J Mol Sci. 2020. PMID: 31973188 Free PMC article. Review.
Cited by
-
Minimal models for cell-cycle control based on competitive inhibition and multisite phosphorylations of Cdk substrates.Biophys J. 2013 Mar 19;104(6):1367-79. doi: 10.1016/j.bpj.2013.02.012. Epub 2013 Mar 19. Biophys J. 2013. PMID: 23528096 Free PMC article.
-
Human retinal organoids release extracellular vesicles that regulate gene expression in target human retinal progenitor cells.Sci Rep. 2021 Oct 26;11(1):21128. doi: 10.1038/s41598-021-00542-w. Sci Rep. 2021. PMID: 34702879 Free PMC article.
-
Protein Phosphatases in G1 Regulation.Int J Mol Sci. 2020 Jan 8;21(2):395. doi: 10.3390/ijms21020395. Int J Mol Sci. 2020. PMID: 31936296 Free PMC article. Review.
-
Prolonged cyclin-dependent kinase inhibition results in septin perturbations during return to growth and mitosis.J Cell Biol. 2018 Jul 2;217(7):2429-2443. doi: 10.1083/jcb.201708153. Epub 2018 May 9. J Cell Biol. 2018. PMID: 29743192 Free PMC article.
-
A hybrid control model for the eukaryotic cell cycle.Nature. 2022 Jun 8. doi: 10.1038/d41586-022-01173-5. Online ahead of print. Nature. 2022. PMID: 35676349 No abstract available.
References
-
- van Werven F. J., Amon A. 2011. Regulation of entry into gametogenesis. Phil. Trans. R. Soc. B. 366, 3521–353110.1098/rstb.2011.0081 (doi:10.1098/rstb.2011.0081) - DOI - DOI - PMC - PubMed
-
- O'Farrell P. H. 2011. Quiescence: early evolutionary origins and universality do not imply uniformity. Phil. Trans. R. Soc. B. 366, 3498–350710.1098/rstb.2011.0079 (doi:10.1098/rstb.2011.0079) - DOI - DOI - PMC - PubMed
-
- Kronja I., Orr-Weaver T. L. 2011. Translational regulation of the cell cycle: when, where, how and why? Phil. Trans. R. Soc. B. 366, 3638–365210.1098/rstb.2011.0084 (doi:10.1098/rstb.2011.0084) - DOI - DOI - PMC - PubMed
-
- Ptacin J. L., Lee S. F., Garner E. C., Toro E., Eckart M., Comolli L. R., Moerner W. E., Shapiro L. 2010. A spindle-like apparatus guides bacterial chromosome segregation. Nat. Cell Biol. 12, 791–79810.1038/ncb2083 (doi:10.1038/ncb2083) - DOI - DOI - PMC - PubMed
-
- Nasmyth K. 1995. Evolution of the cell cycle. Phil. Trans. R Soc. Lond. B 349, 271–28110.1098/rstb.1995.0113 (doi:10.1098/rstb.1995.0113) - DOI - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

