Switches and latches: a biochemical tug-of-war between the kinases and phosphatases that control mitosis
- PMID: 22084385
- PMCID: PMC3203464
- DOI: 10.1098/rstb.2011.0087
Switches and latches: a biochemical tug-of-war between the kinases and phosphatases that control mitosis
Abstract
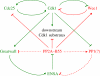
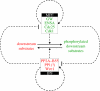
Activation of the cyclin-dependent kinase (Cdk1) cyclin B (CycB) complex (Cdk1:CycB) in mitosis brings about a remarkable extent of protein phosphorylation. Cdk1:CycB activation is switch-like, controlled by two auto-amplification loops--Cdk1:CycB activates its activating phosphatase, Cdc25, and inhibits its inhibiting kinase, Wee1. Recent experimental evidence suggests that parallel to Cdk1:CycB activation during mitosis, there is inhibition of its counteracting phosphatase activity. We argue that the downregulation of the phosphatase is not just a simple latch that suppresses futile cycles of phosphorylation/dephosphorylation during mitosis. Instead, we propose that phosphatase regulation creates coherent feed-forward loops and adds extra amplification loops to the Cdk1:CycB regulatory network, thus forming an integral part of the mitotic switch. These network motifs further strengthen the bistable characteristic of the mitotic switch, which is based on the antagonistic interaction of two groups of proteins: M-phase promoting factors (Cdk1:CycB, Cdc25, Greatwall and Endosulfine/Arpp19) and interphase promoting factors (Wee1, PP2A-B55 and a Greatwall counteracting phosphatase, probably PP1). The bistable character of the switch implies the existence of a CycB threshold for entry into mitosis. The end of G2 phase is determined by the point where CycB level crosses the CycB threshold for Cdk1 activation.
Figures


Similar articles
-
Two Bistable Switches Govern M Phase Entry.Curr Biol. 2016 Dec 19;26(24):3361-3367. doi: 10.1016/j.cub.2016.10.022. Epub 2016 Nov 23. Curr Biol. 2016. PMID: 27889260 Free PMC article.
-
The overlooked greatwall: a new perspective on mitotic control.Open Biol. 2012 Mar;2(3):120023. doi: 10.1098/rsob.120023. Open Biol. 2012. PMID: 22754657 Free PMC article. Review.
-
Two Interlinked Bistable Switches Govern Mitotic Control in Mammalian Cells.Curr Biol. 2018 Dec 3;28(23):3824-3832.e6. doi: 10.1016/j.cub.2018.09.059. Epub 2018 Nov 15. Curr Biol. 2018. PMID: 30449668 Free PMC article.
-
Mitotic progression becomes irreversible in prometaphase and collapses when Wee1 and Cdc25 are inhibited.Mol Biol Cell. 2011 Apr 15;22(8):1191-206. doi: 10.1091/mbc.E10-07-0599. Epub 2011 Feb 16. Mol Biol Cell. 2011. PMID: 21325631 Free PMC article.
-
PP2A function toward mitotic kinases and substrates during the cell cycle.BMB Rep. 2013 Jun;46(6):289-94. doi: 10.5483/bmbrep.2013.46.6.041. BMB Rep. 2013. PMID: 23790971 Free PMC article. Review.
Cited by
-
Phosphorylation: a key regulator of meiosis.Cell Cycle. 2013 Mar 1;12(5):716. doi: 10.4161/cc.23910. Epub 2013 Feb 19. Cell Cycle. 2013. PMID: 23422858 Free PMC article. No abstract available.
-
CDK1-mediated phosphorylation at H2B serine 6 is required for mitotic chromosome segregation.J Cell Biol. 2019 Apr 1;218(4):1164-1181. doi: 10.1083/jcb.201806057. Epub 2019 Feb 14. J Cell Biol. 2019. PMID: 30765437 Free PMC article.
-
Budding yeast greatwall and endosulfines control activity and spatial regulation of PP2A(Cdc55) for timely mitotic progression.PLoS Genet. 2013;9(7):e1003575. doi: 10.1371/journal.pgen.1003575. Epub 2013 Jul 4. PLoS Genet. 2013. PMID: 23861665 Free PMC article.
-
Ryanodine receptor phosphorylation and heart failure: phasing out S2808 and "criminalizing" S2814.Circ Res. 2012 May 25;110(11):1398-402. doi: 10.1161/CIRCRESAHA.112.270876. Circ Res. 2012. PMID: 22628571 Free PMC article. No abstract available.
-
Signaling pathways that regulate cell division.Cold Spring Harb Perspect Biol. 2012 Oct 1;4(10):a005942. doi: 10.1101/cshperspect.a005942. Cold Spring Harb Perspect Biol. 2012. PMID: 23028116 Free PMC article. Review.
References
-
- Masui Y., Markert C. L. 1971. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J. Exp. Zool. 177, 129–14510.1002/jez.1401770202 (doi:10.1002/jez.1401770202) - DOI - DOI - PubMed
-
- Doree M., Labbe J. C., Picard A. 1989. M phase-promoting factor: its identification as the M phase-specific H1 histone kinase and its activation by dephosphorylation. J. Cell Sci. Suppl. 12, 39–51 - PubMed
-
- Lohka M. J., Hayes M. K., Maller J. L. 1988. Purification of maturation-promoting factor, an intracellular regulator of early mitotic events. Proc. Natl Acad. Sci. USA 85, 3009–301310.1073/pnas.85.9.3009 (doi:10.1073/pnas.85.9.3009) - DOI - DOI - PMC - PubMed
-
- Dunphy W. G., Brizuela L., Beach D., Newport J. 1988. The Xenopus cdc2 protein is a component of MPF, a cytoplasmic regulator of mitosis. Cell 54, 423–43110.1016/0092-8674(88)90205-X (doi:10.1016/0092-8674(88)90205-X) - DOI - DOI - PubMed
-
- Gautier J., Minshull J., Lohka M., Glotzer M., Hunt T., Maller J. L. 1990. Cyclin is a component of maturation-promoting factor from Xenopus. Cell 60, 487–49410.1016/0092-8674(90)90599-A (doi:10.1016/0092-8674(90)90599-A) - DOI - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

