Translational regulation of the cell cycle: when, where, how and why?
- PMID: 22084390
- PMCID: PMC3203463
- DOI: 10.1098/rstb.2011.0084
Translational regulation of the cell cycle: when, where, how and why?
Abstract
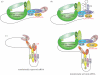
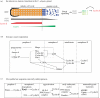
Translational regulation contributes to the control of archetypal and specialized cell cycles, such as the meiotic and early embryonic cycles. Late meiosis and early embryogenesis unfold in the absence of transcription, so they particularly rely on translational repression and activation of stored maternal mRNAs. Here, we present examples of cell cycle regulators that are translationally controlled during different cell cycle and developmental transitions in model organisms ranging from yeast to mouse. Our focus also is on the RNA-binding proteins that affect cell cycle progression by recognizing special features in untranslated regions of mRNAs. Recent research highlights the significance of the cytoplasmic polyadenylation element-binding protein (CPEB). CPEB determines polyadenylation status, and consequently translational efficiency, of its target mRNAs in both transcriptionally active somatic cells as well as in transcriptionally silent mature Xenopus oocytes and early embryos. We discuss the role of CPEB in mediating the translational timing and in some cases spindle-localized translation of critical regulators of Xenopus oogenesis and early embryogenesis. We conclude by outlining potential directions and approaches that may provide further insights into the translational control of the cell cycle.
Figures

References
-
- Suryadinata R., Sadowski M., Sarcevic B. 2010. Control of cell cycle progression by phosphorylation of cyclin-dependent kinase (CDK) substrates. Biosci. Rep. 30, 243–25510.1042/BSR20090171 (doi:10.1042/BSR20090171) - DOI - DOI - PubMed
-
- Lee B., Amon A. 2001. Meiosis: how to create a specialized cell cycle? Curr. Opin. Cell Biol. 13, 770–77710.1016/S0955-0674(00)00282-9 (doi:10.1016/S0955-0674(00)00282-9) - DOI - DOI - PubMed
-
- Groisman I., Jung M. Y., Sarkissian M., Cao Q., Richter J. D. 2002. Translational control of the embryonic cell cycle. Cell 109, 473–48310.1016/S0092-8674(02)00733-X (doi:10.1016/S0092-8674(02)00733-X) - DOI - DOI - PubMed
-
- Lee L. A., Orr-Weaver T. L. 2003. Regulation of cell cycles in Drosophila development: intrinsic and extrinsic cues. Annu. Rev. Genet. 37, 545–57810.1146/annurev.genet.37.110801.143149 (doi:10.1146/annurev.genet.37.110801.143149) - DOI - DOI - PubMed
-
- Bloom J., Cross F. R. 2007. Multiple levels of cyclin specificity in cell-cycle control. Nat. Rev. Mol. Cell Biol. 8, 149–16010.1038/nrm2105 (doi:10.1038/nrm2105) - DOI - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
