Cutting edge: microRNA-181 promotes human NK cell development by regulating Notch signaling
- PMID: 22084432
- PMCID: PMC3237765
- DOI: 10.4049/jimmunol.1100835
Cutting edge: microRNA-181 promotes human NK cell development by regulating Notch signaling
Abstract
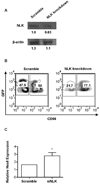
MicroRNAs (miRs) have recently been identified as important regulators of gene expression at the posttranscriptional level. Although it has clearly been established that miRs influence the ontogeny of several immune cell lineages, the role of individual miRs during NK cell development has not been described. In this study, we show that miR-181 expression levels have a profound impact on the development of human NK cells from CD34(+) hematopoietic progenitor cells and IFN-γ production in primary CD56(+) NK cells. We also demonstrate that nemo-like kinase (NLK), an inhibitor of Notch signaling, is a target of miR-181 in NK cells, and knockdown of NLK mirrors the developmental effect of miR-181 overexpression. We conclude that miR-181 promotes NK cell development, at least in part, through the suppression of NLK, providing an important link between miRs and Notch signaling.
Conflict of interest statement
The authors have no financial conflicts.
Figures



References
-
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. - PubMed
-
- O’Connell RM, Rao DS, Chaudhuri AA, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat. Rev. Immunol. 2010;10:111–122. - PubMed
-
- Xiao C, Calado DP, Galler G, Thai TH, Patterson HC, Wang J, Rajewsky N, Bender TP, Rajewsky K. MiR-150 controls B cell differentiation by targeting the transcription factor c-Myb. Cell. 2007;131:146–159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

